Ferroptosis: two-tail fats discovered as the instigators
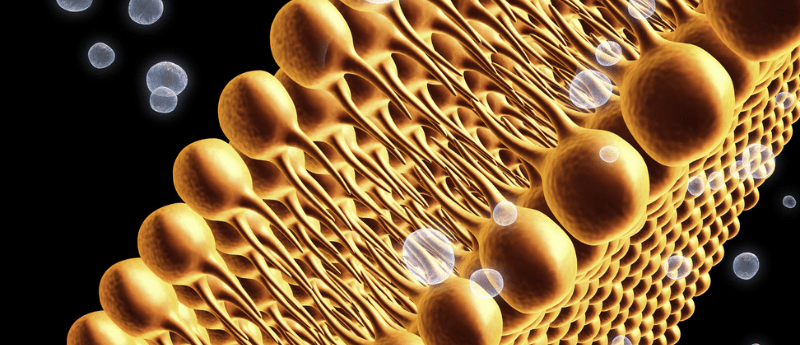
Researchers have uncovered that phospholipids with two polyunsaturated fatty acyl tails play a crucial role in driving ferroptosis.
Ferroptosis is a form of cell death that is dependent on iron. Although it has previously been shown that dietary lipids can modulate ferroptosis, the exact mechanism underlying this hasn’t been uncovered.
A team of researchers from Columbia University (NY, USA) recently investigated two-tail fats (termed PL-PUFA2s or diPUFA phospholipids) in greater detail and showed that this type of lipid interacts with mitochondria to produce reactive oxygen species, which in turn starts a cell death process termed lipid peroxidation.
“The discovery that these diPUFA lipids are important drivers of ferroptosis deepens our understanding of this form of cell death, and these lipids’ role in controlling a cell’s homeostasis in general,” commented study author Brent Stockwell (Columbia University).
 Paralyzing cells could prevent metastasis
Paralyzing cells could prevent metastasis
A mechanism of dynein-powered metastatic locomotion has been identified using soft tissue models, revealing a new clinical target for breast cancer treatment.
By introducing antioxidants that targeted the mitochondria, cellular damage and cell death caused by PL-PUFA2s were halted. Of note, diPUFA lipids were present in aging and Huntington disease-affected brain tissue, connecting it to ferroptosis.
Overall, these findings shed light on the process of ferroptosis in both aging and cancer. Building on the oncology aspect, in a separate paper, another team discovered a novel way in which ferroptosis works involving the gene PHLDA2 through the use of genome-wide CRISPR-Cas9 screenings.
This PHLDA2-mediated ferroptosis also relies on reactive oxygen species and was found to be crucial for the growth of cancerous tissue, even when the usual ferroptosis triggers were not present. These findings suggest that PHLDA2 plays a role in a specific type of ferroptosis that naturally occurs in the body and is important for tumor suppression.
“Harnessing these lipids may eventually help us identify where ferroptosis has occurred and deliberately manipulate them to either induce cell death or stop it. This can begin to give us both understanding and the power to control cell death,” Stockwell added.