Computational modeling takes the guesswork out of developing injectable hydrogels

Researchers have developed a computational framework to predict the properties of granular hydrogel matrices.
A granular hydrogel matrix could be transformative in several biomedical applications including injectable tissue engineering; however, it remains a challenge to design, assemble and optimize these materials. Researchers at the Massachusetts Institute of Technology (MIT) and Harvard University (both MA, USA) have created a computational framework to make it easier to develop injectable hydrogels, which is currently achieved through extensive trial and error.
“These materials have a lot of flexibility and customizability, so there’s a lot of excitement about using them for biomedical applications,” commented Connor Verheyen (MIT), the first author of the study.
A granular matrix is formed when individual hydrogel blocks are compacted together, which acts as a solid or liquid depending on the surrounding environment. This feature makes it a potential material for 3D-bioprinting engineered tissues. They could be designed to encapsulate drugs, biologics and cells for in vitro bioprinting or in vivo tissue engineering to heal or create tissue.
Verheyen wanted to find out how to create a material with reliable and predictable injection properties. This required trial and error to see how changing different features optimized hydrogel structure and mechanical behavior.
“That spurred the effort to take the empirical data, turn it into something that a machine could read and work with, and then ask it to build a predictive map that we could interrogate to help us understand what was going on and how to go to the next step,” explained Verheyen.
 An injectable hydrogel could treat infections following hip replacement surgeries
An injectable hydrogel could treat infections following hip replacement surgeries
An antibacterial hydrogel shows promise as a new minimally invasive treatment option for soft tissue infections around prostheses.
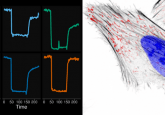
The researchers modeled each stage of the assembly process separately using data from their experiments to create a set of computational models that can predict hydrogel properties.
First, the hydrogel building blocks were created, and the researchers analyzed how the starting material and assembly affected the properties of these. The next stage was compacting the blocks to produce a granular hydrogel network. The size and stiffness of the blocks, the viscosity of interstitial fluid between them and the dimensions of the needle and syringe used to inject the material impacted the properties of the final gel.
This data-driven machine learning model is able to predict the structure, mechanical properties and functional performance of hydrogel materials. The model, along with the data used to create it, is open source and available online.
The researchers can now use this model to predict the best ways to create a hydrogel that can be injected for different applications. They are hoping to develop materials for medical applications, like repairing heart defects, using this approach.