Understanding the endometrium with organoids
A new hydrogel has been developed that can provide an optimal synthetic extracellular matrix (ECM) for more accurate organoid models.
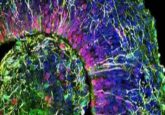
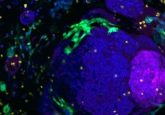
A recent research effort from the Massachusetts Institute of Technology (MIT; MA, USA), led by Linda Griffith, has resulted in the development of a new hydrogel that produces endometrial organoids that successfully replicate the functions and responses of endometrial tissues. This hydrogel will help improve our understanding of this critically underexplored tissue and should help improve therapeutic options for diseases that impact it.
During the menstrual cycle, epithelial cells of the endometrium – the mucosal lining of the uterus – receive signals from sex hormones, translating them into physiological responses in the stromal cells of the tissue through a complex crosstalk between the cells. While the fundamental cycle of hormones and cellular responses is understood, we have a poor interpretation of the cellular mechanisms responsible. As a result, conditions like endometriosis – where endometrial tissue grows in locations outside of the uterus, which can lead to potentially fatal incidences of ectopic pregnancy –are insufficiently understood.
Current organoid models of the endometrium still rely on a hydrogel called Matrigel to provide the foundation for an ECM. Matrigel includes proteins that can interrupt cell signaling, leading to a disruption of the crosstalk between epithelial and stromal cells. As Griffith puts it, “it’s like trying to talk to your friend if you’re standing on the runway at the airport at rush hour.” A noisy model cannot lead to truly clear and definitive insights.
To address this issue, the team set out to develop a new synthetic ECM for the production of more accurate, responsive endometrial organoids. To do this, the team identified the cell-cycle-dependent expression of integrins from endometrial tissue obtained from biopsy as well as the ECM composition. This allowed them to identify optimal candidate peptides to include in the composition of the hydrogel and the cell-matrix interaction cues to use during the development of the organoids.
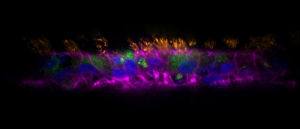 Airway to AirGel: the new in vitro respiratory model
Airway to AirGel: the new in vitro respiratory model
A new organoid model platform has been added to the growing arsenal of tools with which to investigate respiratory conditions.
Initial cultures of epithelial and stromal endometrial cells from human donors in the hydrogel remained intact for 15 days, outperforming those grown in Matrigel, which had begun disintegrating by the 15th day of the experiment.
To test if the matrix produced was capable of establishing operational and accurate endometrial organoids, Griffith and her team treated epithelial and stromal cells, cocultured in the matrix, with synthetic progesterone. This led to the thickening of the epithelial layer, stromal differentiation and an increase in the secretion of the pro-gestational protein prolactin: changes all observed in the in vivo response of the endometrium to progesterone.
To investigate the capability of this synthetic ECM to produce endometrial disease models, the team treated epithelial–stromal cell cocultures with the proinflammatory cytokine interleukin-1β, which is closely associated with endometriosis. This encouraged the proliferation of epithelial cells in the cocultures, a characteristic of endometriosis. The proliferation of the epithelial cells was not observed in epithelial monocultures grown in the same matrix, highlighting that the hydrogel allows for the crosstalk between epithelial and stromal cells observed in vivo.
Commenting on the success and utility of these models, first author Juan Gnecco stated that “with this matrix, we can begin to extrapolate and utilize samples from patients that have been diagnosed with certain reproductive diseases… It really highlights that cell communication is important, not only for sex hormone signaling but even inflammatory communication between cells.” The team is excited to see how this model will impact studies of the endometrium and the conditions that impact it, hopefully leading to improved treatments.