New in vitro model for intestinal mucus
A new in vitro model provides a practical window to investigate intestinal mucus and its response to infection.
Recent research from a team at Stanford University (CA, USA) led by senior authors Gerald Fuller and Sarah Heilshorn, has used cutting-edge cell culture techniques to study intestinal mucus in vitro, avoiding the use of animal models and reducing the disruption to mucus studied. This study could yield a more robust and representative model to study intestinal mucus and subsequently develop therapeutics that could harness the defensive properties of mucus against infection.
Incensed by the cold I have acquired as a result of the UK’s awful winter weather persisting this deep into spring, I have several times today cursed the mucus clogging my sinuses and revolting my colleagues; but, as one of my body’s first-line defenses against infection, my indignation is misdirected. By the miracle of this viscous material’s ability to simultaneously trap potential invaders, provide a protective home for the symbiotic microbes that I rely on for a suite of different functions, and lubricate even more processes, such as digestion, it has likely seen me through some of the worse infections of winter largely unscathed.
But now, it’s in overdrive and I would like to know why. Current scientific approaches to address this question involve the use of animal models, stimulating an infection response before surgically removing the mucus from the location of interest, an often uncomfortable and deeply invasive procedure, particularly in the case of intestinal mucus. What’s more, according to study co-author Margaret Braunreuther, the process of extraction can interfere with the mucus’ properties: “When people collect the mucus from the animal, they’ll pipette it off or scrape it off. But what we found is that when we pipette the mucus layer from our cell culture, we see a very different behavior after that action. We think that the act of pipetting or scraping this very soft polymeric solution is resulting in more liquidlike behavior.”

Icy veins: a new 3D printing method for building artificial blood vessels
Researchers are using ice as a template for the 3D printing of artificial blood vessels in engineered tissues, which could one day be utilized for artificial organ transplants and drug testing.
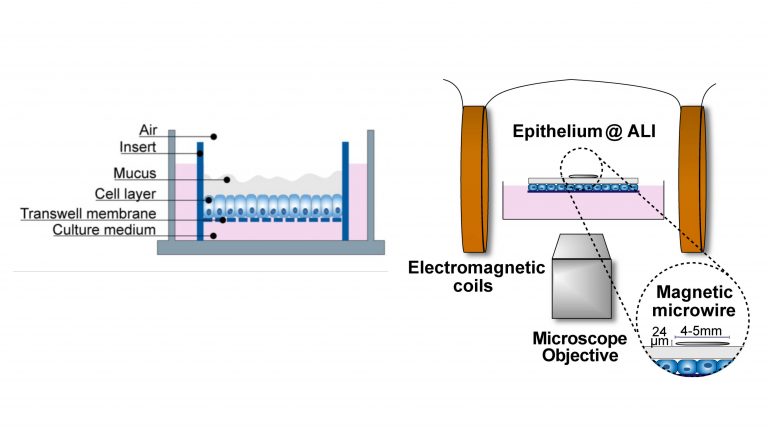
A schematic of the model. Credit: Cai and Braunreuther et al.
To address this limitation and reduce the impact on animal models, the team set out to establish a model that enabled easy access to intestinal mucus, without it needing to be physically manipulated or extracted from an organism. To do this, they grew a layer of intestinal epithelial cells in a lab plate exposed to air, to form an air–liquid interface culture in which the apical surface of the cells – the side that releases the mucus and that would typically face into the lumen of the intestine – was exposed.
To investigate the physical properties of the mucus, they applied a wire to the surface of the mucus layer and subjected it to a magnetic field. By measuring the displacement of the wire in relation to the force applied, the team was able to determine the rheological properties of the mucus. Rheological properties describe the characteristics that dictate the deforming and flowing of a material in response to a force.
As a proof of concept for the model, the team mimicked the infection of the intestine with the parasitic worm Nippostrongylus brasiliensis, applying cytokine IL-13 to the surface to simulate the gut microenvironment during infection with this pathogen. They were then able to observe changes in the properties of the mucus, providing an insight into its response to infection.
Next, the team wants to identify how they can use this model to help improve mucus health and the treatment of infection, while also expanding the model to cover more mucus types. “We have a parallel effort to study airway mucus, looking at conditions such as cystic fibrosis and acute asthma. We also started a collaboration with a group in Berlin to develop drugs to restore a healthy mucus response,” noted Fuller.