Novel hydrogel bioink improves 3D-printed biomaterials
Researchers are one step closer to bioprinting organs and tissues using a novel granular hydrogel bioink.
Every 9 minutes a person is added to the organ transplant waiting list in the United States, according to the Health Resources and Services Administration. 3D-printing organs could be one way of mitigating organ shortages; however, current technologies using hydrogels do not integrate into the body properly and are unable to support cells in thick tissue constructs.
Researchers from Penn State (PA, USA) have achieved previously unattained levels of porosity, shape fidelity and cell integration in a 3D-printed biomaterial using a novel nanoengineered granular bioink. To do so, they created granular hydrogels using self-assembled nanoparticles and microgels, which are discrete hydrogel microparticles.
Bioinks are often based on bulk hydrogels – polymer networks that can absorb large volumes of water whilst retaining their shape and structure. These bioinks have challenges around cell infiltration and migration, which delays or prevents bioink-tissue integration. Additionally, they have nanoscale pores, reducing cell-cell and cell-matrix interactions as well as oxygen and nutrient transfer.
“The main limitation of 3D bioprinting using conventional bulk hydrogel bioinks is the trade-off between shape fidelity and cell viability, which is regulated by hydrogel stiffness and porosity,” Amir Sheikhi, the corresponding author of the study, explained. “Increasing hydrogel stiffness improves the construct shape fidelity, but it also reduces porosity, compromising cell viability.”
 Step aside 3D printing, there’s a new technique for biofabricating a heart
Step aside 3D printing, there’s a new technique for biofabricating a heart
The first biohybrid model of human ventricles has been used to answer a centuries-old question.
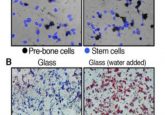
Recently, researchers have turned to microgels to create tissue-engineered scaffolds finding that they can decouple the stiffness of hydrogels from their porosity, as well as regulate the porosity. These granular hydrogel scaffolds can also form 3D constructs in situ. However, to achieve these beneficial characteristics, the microgels needed to be tightly packed together, negatively impacting porosity and reducing cell viability and motility.
The researchers addressed these issues by increasing the stickiness of microgels to each other, removing the need for tight packing and, therefore, preserving the porosity. They achieved this by reversibly binding the microgels using nanoparticles that self-assemble. The resulting granular hydrogel bioink had well-preserved microporosity, enhanced shape fidelity and printability.
“Our work is based on the premise that nanoparticles can adsorb onto polymeric microgel surfaces and reversibly adhere the microgels to each other, while not filling the pores among the microgels,” explained Sheikhi. “The reversible adhesion mechanism is based on heterogeneously charged nanoparticles that can impact dynamic bonding to loosely packed microgels. Such dynamic bonds may form or break upon release or exertion of shear force, enabling 3D bioprintability of microgel suspensions without densely packing them.”
By addressing the challenges of current 3D bioprinting, this research could open new doors in tissue engineering and regeneration and in situ 3D printing of functioning organs.