Step aside 3D printing, there’s a new technique for biofabricating a heart
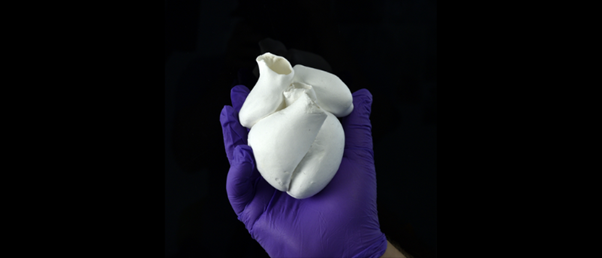
The first biohybrid model of human ventricles has been used to answer a centuries-old question.
Unlike other organs, the heart cannot repair itself after injury, so being able to engineer an entire heart for transplant is important for the future of cardiac medicine. To build a heart from scratch, researchers would need to replicate the unique structures that make up this organ, including the helical geometries that result in a twisting motion when the heart beats.
In 1969, Edward Sallin, the former chair of the Department of Biomathematics at the University of Alabama Birmingham Medical School (AL, USA), theorized that this helical alignment was needed to achieve large ejection fraction – the percentage of how much blood the ventricle pumps with each contraction. The purpose of these spiraling muscles has been challenging to study partly because fabricating a heart, let alone one with different geometries, is difficult.
Now, more than 50 years later, bioengineers from the Harvard John A. Paulson School of Engineering and Applied Sciences (MA, USA) have made a biohybrid model of human ventricles and found that this helical alignment does indeed increase the amount of blood pumped by the ventricle with each contraction.
“Our goal was to build a model where we could test Sallin’s hypothesis and study the relative importance of the heart’s helical structure,” said John Zimmerman, co-first author of the paper.
 Biochemists find a way to mend a broken heart
Biochemists find a way to mend a broken heart
Novel technology returns cardiomyocytes to a stem cell-like state to promote their proliferation and regeneration in an in vitro study.
Building a ventricle was made possible using a novel method called Focused Rotary Jet Spinning (FRJS), a type of additive textile manufacturing, which was developed by the same research group led by Kit Parker. This is a high-throughput fabrication method that forms a scaffolding that controls tissue alignment and, once seeded with cardiomyocyte cells, forms tissue-engineered ventricles.
FRJS works like a candyfloss machine, with a liquid polymer solution loaded into it instead of sugar. The device then spins creating centrifugal force and pushes the polymer out through a small opening. The solvent evaporates as the polymer leaves the candyfloss-like machine, so the polymer solidifies and forms a fiber. As the polymer fiber is deposited onto a collector, a focused airstream controls the orientation. If the angle of the collector is changed, the fibers twist resulting in helical structures similar to those found in our heart muscle.
“The human heart actually has multiple layers of helically aligned muscles with different angles of alignment,” said Huibin Chang, another co-first author. “With FRJS, we can recreate those complex structures in a really precise way, forming single and even four-chambered ventricle structures.”
FRJS can spin fibers in the single micron scale, which is 50-times smaller than a human hair, much quicker than 3D printing methods that perform slower as features get smaller. This is essential when building a heart because, for instance, collagen is about a single micron in diameter. For a 3D printer, it would take more than 100 years to print every piece of collagen in the human heart, whereas the FRJS can do it in one day.
After making the ventricle, the researchers seeded them with either rat cardiomyocyte cells or cardiomyocytes derived from human stem cells. Around a week later, several thin layers of tissue covered the scaffold and followed its alignment. The researchers reported that the resulting beating ventricles performed the same twisting motion as human hearts.
Using this technique, the researchers made ventricles with helically aligned fibers and circumferentially aligned fibers. They compared the ventricle deformation, speed of electrical signaling and ejection fraction between the ventricles and found that on all accounts, the helically aligned tissue performed best.
“Since 2003, our group has worked to understand the structure-function relationships of the heart and how disease pathologically compromises these relationships,” Parker concluded. “In this case, we went back to address a never-tested observation about the helical structure of the laminar architecture of the heart. Fortunately, Professor Sallin published a theoretical prediction more than a half century ago and we were able to build a new manufacturing platform that enabled us to test his hypothesis and address this centuries-old question.”





