The cellular hitman turned biosensor
Researchers have hijacked a bacterial toxin in a bid to understand how its membrane-spanning capabilities can be utilized to create a nanopore biosensor.
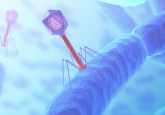
Artificial or organic biosensors are utilized for the detection of biological molecules, with particular use in the application of both DNA and RNA sequencing. In recent years, attention has turned towards pore-forming toxins (PFTs) for use as biosensors. PFTs are specialized proteins released by bacteria that create pores in cell membranes, thereby inducing apoptosis.
The pore-forming capabilities of PFTs make them ideal for use in the creation of biosensors. Biological molecules pass through the pore, creating electrical signals that can provide information regarding the nature of the molecule.
Led by Matteo Dal Peraro, a team of researchers from the Swiss Federal Institute of Technology Lausanne (Switzerland) focused on aerolysin, a well characterized PFT that is produced by the bacterium Aeromonas hydrophila. Aerolysin forms incredibly narrow pores, resulting in a higher level resolution when characterizing biological molecules.
Previous studies have demonstrated the “sensing” abilities of aerolysin; in this work, the team sought to understand it’s structural properties. Computer simulations were utilized to model the structure of aerolysin, providing insight into how the constituent amino acids affected functionality. The model was then used to predict the relative impact of various strategic amino acid changes.
- Organic nanotubes could change the delivery of targeted cancer treatments
- Nano-sized laser: the future for imaging living tissues?
- Child’s play: how a children’s toy inspired a wearable respiration sensor
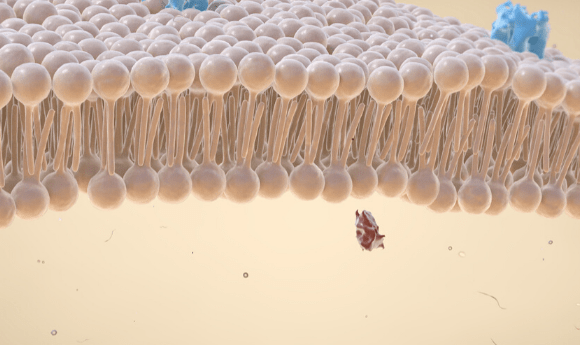
Utilizing the information gained from the computational simulation, lead author Chan Cao created 16 genetically engineered aerolysin pores. Each mutant pore was then embedded into a lipid bilayer to replicate the conditions of a cell membrane. Measurements were taken to compare how different molecular changes impacted the function of aerolysin.
The researchers demonstrated that the cap-like structure of aerolysin is vital for its normal function. The electrostatic properties of the cap control the tethering of the desired molecule and mediates its journey through the pore.
“By understanding the details of how the structure of the aerolysin pore connects to its function, we can now engineer custom pores for various sensing applications,” explained Dal Peraro. “These would open new, unexplored opportunities to sequence biomolecules as DNA, proteins and their post-translational modifications with promising applications in gene sequencing and biomarkers detection for diagnostics.”
Understanding the structural properties of aerolysin opens the possibility of engineering single-site mutants for the creation of specialized nanopore biosensors. A patent has been filed for the information pertaining to the characterization of genetically engineered aerolysin.