What is the role of epigenetics in cancer development?
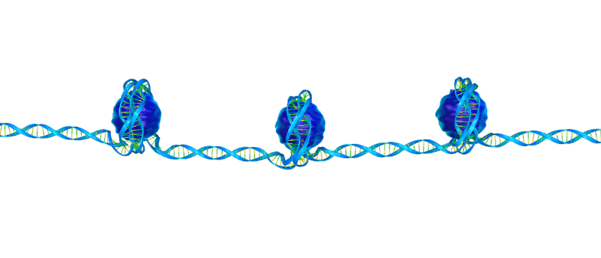
Epigenetics may have more control over cancer development than previously thought, according to two papers published simultaneously.
An international collaboration of researchers at The Institute of Cancer Research (London, UK), Human Technopole (Milan, Italy) and Queen Mary University of London (UK) is responsible for two studies that highlight the importance of epigenetics in bowel cancer progression. By further understanding the genetic and epigenetic causes of cancer, researchers can provide insight into more nuanced treatment options as well as offer the ability to predict how someone will respond to treatment.
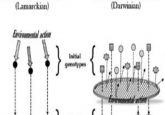
Epigenetics describes the process by which the physical structure of DNA is altered without affecting the actual genetic code. However, altering the 3D structure can control gene accessibility and, in turn, expression. Once believed to be a product of gene mutations alone, cancer development is now understood to be affected by epigenetics as well.
This group of researchers has successfully been able to monitor the epigenetic influences on how colorectal cancers grow and evolve, separate from but parallel to DNA mutations; they found that epigenetic changes have significant control over a cancer’s ability to evolve.
“We have for the first time been able to map epigenetic changes alongside the accumulation of DNA mutations as a colorectal tumor evolves. This provides exciting opportunities to create new treatments for cancer that don’t target the effects of DNA mutations, but instead the epigenetic changes which determine how genes are read,” expressed Andrea Sottoriva (Human Technopole), co-senior author of the study.
 Nanocarriers deliver 2-in-1 cancer treatment
Nanocarriers deliver 2-in-1 cancer treatment
Researchers develop an innovative nanocarrier to simultaneously deliver a chemotherapeutic and a drug to support the immune system.
The first of the two studies used spatial multiomic profiling of colorectal tumors at single-clone resolution to compare the effects of the genome and epigenome on cancer characteristics [1]. The team collected 1,370 samples from 30 patients with colorectal cancer; from these, they were able to construct 527 whole genomes and 297 whole transcriptomes. They found that epigenetic changes are common in cancerous cells, can be inherited following cell division, influence DNA mutation accumulation and can give cancer cells survival advantages.
The second of the papers used spatially resolved paired whole-genome and transcriptome sequencing to investigate how intratumor gene expression varies [2]. In other words, they wanted to establish why one cell can act so differently from another cell within the same tumor. When comparing the DNA sequences of different areas of the same tumor, they found that DNA mutations were rarely associated with changes in gene activity. Additionally, they observed that cancerous cell behavior was often controlled by factors independent of DNA mutations.
The researchers stressed that their findings are observational and require further research to understand the relationship between epigenetics and cancer behavior. However, these studies do provide a strong base upon which this further research can be conducted.
Co-senior author of the study, Trevor Graham, concluded, “I hope our work will change the way we think about cancer and its treatment – and should ultimately affect the way patients are treated. Genetic testing for cancer mutations only gives us part of the picture about a person’s cancer – and is blind to ‘epigenetic’ changes to how genes are read. By testing for both genetic and epigenetic changes, we could, potentially, much more accurately predict which treatments will work best for a particular person’s cancer.”