A surprising new suppressor
Genetically altered SHANK proteins are linked to autism. A new study shows SHANKs play a powerful, unexpected role in cancer as well.
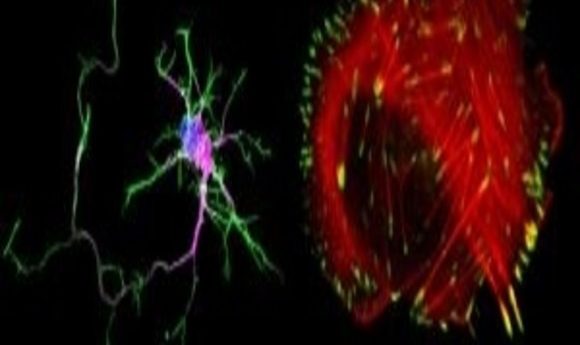
SHANK regulates adhesion and protrusion in cancer cells and neurons. The left image shows a bone cancer cell stained for Actin (red) and paxillin (green) and the right image shows a primary rat hippocampal neuron stained for actin (green) Dapi (blue) and MAP2 (purple).
Credit: Guillaume Jacquemet at the Turku Centre for Biotechnology
Integrins are ubiquitous adhesion receptors that are at the center of countless biological processes. They attach a cell to the extracellular matrix and are key players in signal transduction. When they are activated inappropriately, cancer can result.
Although scientists know a lot about how integrins are activated, they know little about what inhibits them. Now, a recent paper in Nature Cell Biology uncovers a new, unexpected suppressor of integrin: SHANK, a protein linked to autism.
“SHANK was always a mysterious protein because it’s fairly ubiquitous. It expresses in all the tissues, and so far it has been associated mostly with neuronal activity,” said Igor Barsukov of the University of Liverpool, co-lead investigator of the study. “But this study gives a different view of what SHANK functions might be in neuronal and non-neuronal cells.”
Barsukov is a structural biologist who studies talin, a protein that binds and activates integrin. Back in 2013, after publishing a paper on the structure of talins, he received a call from Hans-Juergen Kreienkamp, at University Medical Center Hamburg-Eppendorf in Germany, who told Barsukov that the sequence of the N-terminal part of SHANK3 was very similar to the talin domain they had recently identified. At that time, this region of SHANK3 was thought to be unstructured and not important for the protein’s function.
Curious, Barsukov solved the structure of SHANK’s N-terminal region and was surprised to find that it contained a previously undetected Ras-association SPN domain with a high affinity for GTP-bound Ras and Rap G-proteins. Rap1 signaling triggers integrin activation.
Separately, Johanna Ivaska at the University of Turku in Finland, who studies integrin activity in cells, found that knocking down SHANK increases integrin activation though an unknown mechanism. Joining forces, the team found that the SHANK SPN domain sequesters the Rap1 and R-Ras proteins, reducing their bioavailability at the plasma membrane and blocking their integrin activation activity, which prevents activation of integrin.
When the researchers suppressed SHANK3, Rap1 activity increased at the plasma membrane, as did spreading, migration, and invasion of cells, which could contribute to cancer progression and metastasis.
“The exciting thing for me about this paper was we came from different backgrounds, trying to answer questions looking at this protein in different environments and at different angles, and eventually found the mechanism that we didn’t really expect to discover initially,” said Barsukov.
There are several mutations in the SHANK3 SPN domain that are linked to autism in a small number of patients; this study showed that these mutations disrupt integrin inactivation by SHANK3 through the RAP1-RIAM-talin axis in cancer cells and neurons. This direct link of SHANK to the Rap and Ras signaling pathways may also have integrin-independent functions in neuronal cells.
“Targeting SPN domain with small molecules to disrupt SHANK interactions could potentially have practical implications,” said Barsukov.