Antibody–drug conjugates: a breakthrough in the targeted therapy era
 Grace Liu (left) is a Technical Account Manager at Sino Biological US Inc. (PA, USA). Grace joined Sino Biological in 2022, supporting CRO services and project management in the western and central US regions. Prior to joining Sino Biological, she worked in the Houston Methodist Research Institute (TX, USA) as a postdoctoral fellow focused on sustained drug-eluting devices for cancer immunotherapy.
Grace Liu (left) is a Technical Account Manager at Sino Biological US Inc. (PA, USA). Grace joined Sino Biological in 2022, supporting CRO services and project management in the western and central US regions. Prior to joining Sino Biological, she worked in the Houston Methodist Research Institute (TX, USA) as a postdoctoral fellow focused on sustained drug-eluting devices for cancer immunotherapy.
In this interview, Grace walks us through antibody–drug conjugate (ADC) development, highlighting their structure, their therapeutic potential and the solutions Sino Biological offers to advance ADC development.
What are ADCs?
ADCs are a promising cancer treatment modality designed to deliver highly potent cytotoxic agents directly to tumor cells, thereby maximizing therapeutic efficacy and minimizing systemic toxicity.
Conventional cancer chemotherapy is often limited by a narrow therapeutic window due to its poor specificity towards tumor cells. Consequently, chemotherapeutic agents commonly damage normal cells with high mitotic rates, leading to an array of adverse effects. More selective therapeutics are needed. Known as ‘magic bullets’, the ADC comprises a monoclonal antibody (mAb, used as the carrier) tethered to a cytotoxic drug (known as the payload) via a chemical linker. ADCs take advantage of the specificity of the mAb towards a tumor target antigen to achieve targeted delivery of the cytotoxic drug to tumor cells.
Once the ADC binds to its target antigen on the tumor cell, the ADC–antigen complex is internalized through receptor-mediated endocytosis into an early endosome, which subsequently matures and fuses with a lysosome. The ADC releases its payload in the lysosome, and the free, active payload then diffuses into the cytoplasm and exerts its cytotoxic effects, leading to cell death.
What are the key components of ADCs?
ADCs consist of three main elements: a mAb backbone, a cytotoxic payload and a linker that binds them. The creation of ADCs goes beyond a simple combination, and the key challenge is balancing efficacy and safety.
mAb: the precision guide in ADC therapy
Acting as the tumor-targeting vehicle, the mAb component of the ADC should possess high antigen specificity, strong antigen-binding affinity and efficient internalization, among other properties. mAbs recognizing antigens that are predominantly overexpressed on the surface of tumor cells are preferable for ADCs, ensuring the tumor-specific delivery of payloads and a wide therapeutic window. Target antigens of the approved ADCs include HER2, TROP2, Nectin-4, EGFR, TF and FRα for solid tumors and CD19, CD22, CD33, CD30, BCMA and CD79b for hematologic malignancies. Internalization efficiency has important implications for the efficacy of ADCs. Higher antigen-binding affinity of the mAb often contributes to more rapid internalization. However, excessively strong binding can in turn hinder the penetration of ADCs into solid tumors, known as the binding site barrier effect. Therefore, various parameters must be carefully considered and balanced to ensure the optimal mAb design for ADCs.
Desirable characteristics of the antibody moiety of ADCs should also include minimal immunogenicity and long circulating half-life. Humanized and fully human antibodies, significantly less immunogenic than murine and chimeric mAbs, are most commonly employed as the ADC backbone. IgG1 subtype is preferentially used due to its induction of strong effector functions and stability in the systemic circulation with a half-life of 21 days.
Linker: a balancing bridge
The linker connects the cytotoxic payload to the mAb. Its purpose is twofold: (1) affording sufficient stability when ADCs circulate in plasma to prevent premature drug release and the resulting systemic toxicity; and (2) enabling efficient release of the payload upon internalization within tumor cells. Currently available linkers fall into two primary categories: cleavable and non-cleavable. Cleavable linkers are designed to break down in response to tumor-associated factors and can be further categorized into acid-labile linkers (e.g., hydrazones), reducible/glutathione-sensitive linkers (e.g., disulfides) and protease-sensitive/peptide linkers (e.g., cathepsin-sensitive valine–citrulline dipeptides). Non-cleavable linkers depend on complete degradation of the mAb in lysosomes, resulting in the liberation of the cytotoxic cargo that remains linked to an amino acid residue (typically a cysteine or lysine) from the degraded mAb. Examples of non-cleavable linkers include thioether linkers and maleimide-based linkers.
Non-cleavable linkers are characterized by greater stability in plasma, thus resulting in reduced off-target toxicity. However, this strategy ultimately releases an amino acid–linker–cytotoxin construct, which can potentially hinder cytotoxicity of the payload, restricting the applicable cytotoxic drugs to those exerting antitumor effects despite being chemically modified. By contrast, cleavable linkers are cleaved in response to environmental differences between circulation and tumor cells, enabling their compatibility with a wider range of payloads and allowing diversified mechanisms of action. They can achieve efficient, controlled release of active payloads. However, a downside of their flexibility in real-world use is the potential risk of premature drug release in circulation and consequent systemic toxicity. Therefore, when it comes to linker chemistry, careful design that strikes a balance between the stability of ADCs in circulation and the efficiency of payload release is crucial. Cleavable linker strategy currently dominates the ADC landscape – most of the approved drugs and nearly all ADCs in clinical trials exploit cleavable linkers, and in most cases, enzyme-cleavable linkers.
Payload: the ADC warhead
As the ultimate effector component of ADCs, the payloads are typically highly potent cytotoxic drugs with half-maximal inhibitory concentration (IC50) values at sub-nanomolar or picomolar levels, as opposed to the micromolar IC50 range of conventional chemotherapeutic agents. The high potency of payloads is crucial to achieve therapeutic efficacy since only approximately 2% of the administered ADC dose will reach the tumor site and individual mAbs can accommodate relatively few payload molecules. The payloads currently used for ADCs are essentially represented by two categories: (1) tubulin inhibitors and (2) DNA-damaging agents. The first class disrupts microtubule assembly, inducing mitotic arrest, and consequently, cell death, such as auristatins (e.g., MMAE and MMAF) and maytansinoids (e.g., DM1 and DM4). The second class of payloads includes calicheamicins inducing DNA double-strand breaks, pyrrolobenzodiazepines (PBDs) producing DNA cross-links, duocarmycins causing DNA alkylation, and camptothecins (e.g., DXd and SN-38) inhibiting topoisomerase I (TOPO1) and hence leading to DNA breaks. Beyond these cytotoxins at the forefront of ADC development, novel payloads including immune stimulants (e.g., STING agonists and TLR agonists) and proteolysis-targeting chimeras (PROTACs) are emerging.
Drug-to-antibody ratio (DAR) – the average number of payload moieties attached to each mAb – is a key parameter for ADC design. Theoretically, high-DAR ADCs would be expected to be more potent. However, they tend to demonstrate excessive hydrophobicity, inducing antibody aggregation, accelerated plasma clearance and unwanted toxicity in vivo. As such, fine-tuning the DAR to balance efficacy and toxicity is crucial. In addition to an optimal DAR, determinants of ADC efficacy include favorable features of payloads such as amenability to conjugation, proper aqueous solubility and superior stability in aqueous formulation as conjugates.
Why do ADCs attract attention in healthcare?
Marking a breakthrough in the targeted therapy era, ADCs hold a great deal of promise to meet the increasing demand for enhanced efficacy and safety in cancer treatment. The ADC is designed to be a ‘targeted chemotherapy’ that holds the chemotherapy and the mAb together to target efficacy to the tumor. This approach for targeted payload delivery works and has attracted widespread attention for its clinical benefits and significant advantages over conventional chemotherapy. For example, ADCs can potentially improve objective response rates and durability of responses in both liquid and solid tumor indications as single agents or in combination with chemotherapies or checkpoint inhibitors. Furthermore, as personalized medicine becomes more tailored with a wealth of individualized data, ADCs with the potential to target specific cancer subtypes could become an increasingly powerful tool to combat cancer.
Nevertheless, ADCs still face many obstacles, ranging from difficulties in selection and validation of appropriate target antigens, complex design challenges, cost-intensive and time-consuming development and production to issues with linker stability, ADC heterogeneity, toxicity and resistance. The right combination of antibody optimization, linker design, conjugation chemistry and payload class, as well as a better understanding of cancer biology and the target landscape, are essential for developing more effective and safer ADCs to enhance patient outcomes. Extensive efforts are ongoing.
Overall, the targeted nature, efficacy, personalized approach, innovation and market potential of ADCs as well as the huge room for improvement have made them a compelling area of research and development in healthcare, attracting attention from various stakeholders in the field.
What’s the current status of ADCs in therapeutics?
In 2000, gemtuzumab ozogamicin (Mylotarg®) became the first ADC to be approved by the US FDA for acute myeloid leukemia. As of April 2024, there were 13 clinically approved ADCs worldwide for the treatment of various hematologic malignancies and solid tumors [1]. Additionally, there are more than 100 ADCs undergoing evaluation in clinical trials across a broader set of cancers, with many of them already demonstrating remarkable success in advanced phases [2].
Despite the successes to date and the promising outlook, hurdles to tackling tumor heterogeneity, drug resistance and treatment-related adverse effects remain in the field of ADCs. Advancements in ADC technology and a more nuanced mechanistic understanding of ADC activity are among the key drivers to boost next-generation ADCs with improved targeting, potency and safety profiles. Bispecific ADCs are emerging in the realm of next-generation ADCs and can be categorized into two types to date: (1) bispecific ADCs that target two different antigens; and (2) biparatopic ADCs that recognize two distinct epitopes of the same antigen. The first class represents a strategy aiming to improve ADC selectivity for tumor versus normal tissue and kill a broader spectrum of tumor cells when the targets are heterogeneously expressed within the tumor. The second strategy seeks to enhance ADC internalization and trafficking to lysosomes to maximize payload delivery into tumor cells.
Beyond cancer, there is growing interest in exploring the potential applications of ADCs for autoimmune diseases, bacterial infections and more non-oncological indications. For example, arming antibodies with the anti-inflammatory agent glucocorticoids results in selective delivery of the drug to stimulated immune cells, dampening inflammatory responses while minimizing side effects in the treatment of inflammatory diseases. Moreover, antibody–antibiotic conjugates (AACs) are designed to effectively eliminate intracellular bacteria and are exploited as a therapeutic platform with the potential to enhance antibiotic efficacy against hard-to-treat bacterial infections.
How is Sino Biological involved in advancing the development of ADCs?
The development of ADCs involves a complex process where factors including the target, antibody, payload and linker must be carefully considered. Furthermore, due to their structural diversity and complexity, ADCs encounter unique challenges in formulation, manufacturing and quality control.
As a prominent supplier in the pharmaceutical industry, Sino Biological is deeply engaged in every crucial aspect of ADC development. We offer comprehensive ADC development solutions tailored to the specific needs of pharmaceutical and biotech companies, covering the entire journey from early discovery to clinical studies (Figure 1). Our services are crafted to assist clients in expediting their progress throughout the ADC development process.
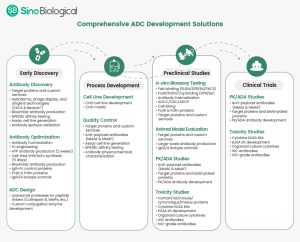
Figure 1. ADC development solutions at Sino Biological.
The opinions expressed in this interview are those of the interviewee and do not necessarily reflect the views of BioTechniques or Taylor & Francis Group.
This article was supported by Sino Biological.