Hitting cancer where it’s vulnerable

The genetic defects that cause cancer offer potential therapeutic targets. Now, researchers are targeting some of these genes—those essential to cell survival—to specifically kill cancer cells.

SF3B1 is part of the U2 snRNP spliceosome complex—a protein complex necessary for proper gene expression. Cancer cells with partial SF3B1 loss die when SF3B1 is moderately suppressed.
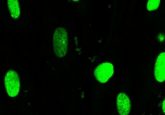
Image courtesy of Brenton Paolella.
Despite years of research, many of the mutated proteins known to cause cancer remain undruggable. CYCLOPS genes (Copy-number alterations Yielding Cancer Liabilities Owing to Partial losS)—or genes where one of two copies of a gene essential to cell survival is lost—present a vulnerable target for killing cancer cells. Now, a recent study in eLife identifies CYCLOPS genes present in several cancers and characterizes the mechanism of one such gene, SF3B1, for potential tumor treatment (1).
Researchers first speculated about gene copy-loss as a cancer vulnerability in 1994. However, according to Rameen Beroukhim, from the Dana-Farber Cancer Institute and lead author of the study, technology limitations prevented researchers from confirming that CYCLOPS genes existed until after 2010 when genome-wide screens verified cancer cell dependencies—or which genes known to be altered in cancer cells that are vital to survival.
“We know now that this CYCLOPS mechanism is prevalent, a major determinant of dependencies in cancer cells, and that it’s therapeutically relevant,” said Beroukhim. “This is something we wanted to study in more depth.”
Beroukhim and his team integrated several databases containing genome-wide information to identify cancer cell dependencies in 179 cell lines. “The typical cancer cell has altered approximately one-third of its genome by copy number alterations,” said Beroukhim. Of these genes, 124 are CYCLOPS genes found across different cancer types.
One of the most potent CYCLOPS genes is SF3B1. As part of the spliceosome—a protein complex necessary for proper gene expression—SF3B1 protein is known to be essential for cell survival but has not been explored as a potential cancer target. The researchers established the vulnerability of cells with partial SF3B1 loss by moderately suppressing SF3B1 in human cancer cells. Indeed, cells with partial SF3B1 loss died due to defective splicing.
The team worried that suppressing essential genes might be a counterproductive therapeutic approach since it would also hurt healthy cells. However, healthy cells with both copies of SF3B1survived limited SF3B1 suppression. “The first confirmation of the phenotype was very clear. You can kind of breathe after that,” said William Gibson, co-first author of the study.
The ability to selectively kill cancer cells missing a copy of SF3B1 by gently suppressing SF3B1 presents a potential cancer therapy. However, Gibson noted that this project isn’t really about SF3B1. “The CYCLOPS idea…allows you to have so many possible different targets in these tumors that have previously been undruggable,” he said
“The practicalities of trying to go out and translate CYCLOPS gene dependencies into therapeutic approaches is really the big central and exciting question,” said Brenton Paolella, co-first author of the study. “It opens up a whole new field of study,” added Beroukhim.
Xiongbin Lu from The University of Texas MD Anderson Cancer Center, who was not involved in this study, agrees. “We can actually use some of the results from this [study] to validate CYCLOPS genes in each type of cancer for specific cancer therapies,” he said.