Jetlag and obesity share a path to cancer
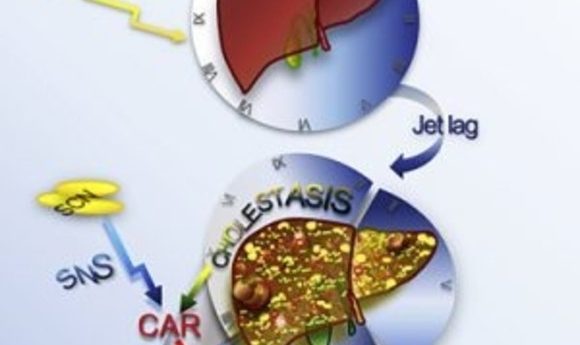
Chronic sleep disruption causes deleterious effects on liver function, just like obesity.
Obesity is a pandemic in Western cultures, burgeoning faster than researchers can understand its multilevel impact. Alongside metabolic diseases, liver cancer is significantly increasing among obese individuals. In fact, liver cancer is the fastest growing cause of cancer-related deaths in the US. But obesity doesn’t carry the sole responsibility for this.
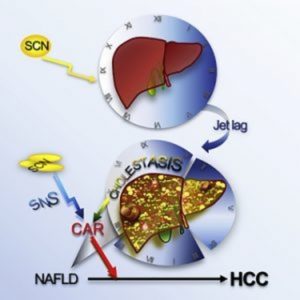
Another ubiquitous, yet more silent, epidemic is quickly integrating itself into the everyday fabric of our society: sleep disruption—or “social jetlag”—where our sleep cycles and social cycles clash, resulting in sleep loss (1). Jetlag is a known risk factor for obesity, and like obesity, it shows links to liver cancer. Now, in a new study in the journal Cancer Cell, researchers unveil a pathway to liver cancer shared by jetlag and obesity (2).
“Our circadian clock is the master regulator of our physiology,” said Loning Fu, an associate professor at Baylor College of Medicine and senior author of the study. “I think if anyone tried to understand the mechanism of cancer from a physiology point of view, one should understand the clock.”
To model jetlag in mice, researchers exposed wild-type mice to variations in daily light/dark cycles that deviated from the normal 24-hour light/dark cycles used for controls. By 90 weeks of age, 96% of the jetlagged mice, compared to 20% of controls, developed non-alcoholic fatty liver disease (NAFLD); liver cancer arose in 9% of the mice.
The jetlagged mice not only built up fat in their livers but also showed increased bile acid production. “Bile acid is a biodetergent and can actually cause membrane damage, which is a very important endogenous mechanism that leads to liver damage,” said Fu.
To unravel the mechanisms underlying the increase in fat and bile acid production, the researchers carried out a large-scale microarray analysis of total liver RNA. They found substantial gene deregulation in the jetlagged mice similar to what is seen in cases of human liver cancer. Among the top deregulated pathways were those featuring two nuclear receptors, farnesoid X receptor (FXR) and constitutive androstane receptor (CAR), which are involved in liver bile acid production and function, respectively. Thus, as seen in obesity, jetlag also increases bile acid production in the liver by suppressing FXR, and this causes activation of CARs.
Jetlagged mice lacking the CAR did not show an increase in downstream inflammatory or oncogene pathways and exhibited a resistance to liver cancer. Fu’s group is currently testing pharmacological inhibitors of CAR as potential treatments for reversing or preventing jetlag-induced liver cancer.