Two tales that end in blood
Refer a colleague
In a major breakthrough for stem cell therapy, two research groups generated blood stem cells using different approaches. Which is the best?
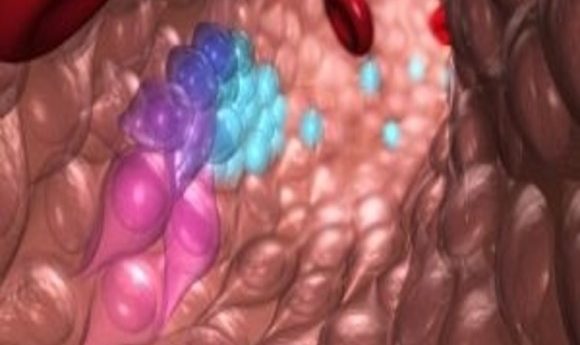
This illustration depicts blood stem and progenitor cells emerging from hemogenic endothelial cells during normal embryonic development. The gradation of purple-blue (from purple to blue) cells indicates the transition from hemogenic endothelium to hematopoietic/progenitor cells. The red cells are red blood cells.
Credit: O’Reilly Science Art
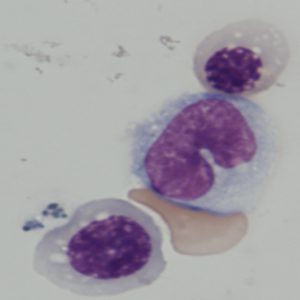
Blood cells derived from iPS cells in George Daley’s lab.
Credit: Rio Sugimura, Harvard Medical School
Patients suffering from leukemia die when abnormal blood cells take over their healthy blood cells. Importantly, their hematopoetic stem cells, which produce mature blood cells in the bone marrow are incapable of producing normal blood cells. These patients can be saved by transplanting blood-generating stem cells from the bone marrow of a healthy donor, which could regenerate their blood cells. Given the recent advances in stem cell research, this sounds doable, but the main challenge is finding a donor whose blood type perfectly matches that of the patient. Thousands of leukemia patients who cannot find a matching donor still die each year.
Now, two groups have independently generated hematopoetic stem cells in the lab, albeit using different approaches. Their methods, published in Nature, may potentially solve donor-dependence issues and facilitate easy availability of hematopoetic stem cells for therapeutic options in the future.
“These two papers provide an alternative source that has not been used before,” said Mick Bhatia from the McMaster Cancer and Stem Cell Biology Research Institute in Hamilton, Canada, who was not involved in either of the studies. Bhatia and other stem cell researchers have been trying for more than a decade to figure out the best way to produce hematopoetic stem cells in the lab. “That really hasn’t been achieved until now.”
Keeping Traditions Alive
For one of the new papers, George Daley from Harvard Medical School led a team to generate hematopoetic stem cells using a multistep approach based on a conventional method [1]. Daley’s team first genetically modified human skin cells to produce pluripotent stem cells (iPS), which are similar to embryonic stem cells in that they can generate different types of cells. Next, the team chemically manipulated the iPS cells to generate cells that develop into hematopoetic stem cells in culture using established methods. Finally, they injected these modified cells into animals to develop functional hematopoetic cells, a step that stem cell researchers had previously failed to accomplish.
“It’s a very complex procedure. It requires a lot of steps, you know, to mimic what embryonic development does,” Daley explained. “It just took a long time to figure all that out.”
His team achieved their goal by forcing overexpression of seven transcription factors that regulate expression of other genes in the modified cells. Then they injected these cells into mice that were irradiated to weaken their immune systems. If the injected cells develop into functional hematopoetic cells, they produce immune cells, along with blood cells, keeping the mice alive. The team noted that these mice showed engraftment, where hematopoetic stem cells generated blood cells and immune cells. Daley’s approach, thus, successfully produced hematopoetic-like stem cells, which showed similar—but not identical—properties to those of natural blood stem cells.
Think Outside the Box, but Within the Niche
“The problem with that [approach] is that you have to get fibroblasts, you need to get iPS, and then you have put more transcription factors, and the engraftment was very poor,” noted Shahin Rafii, professor at Weill Cornell Medical College, who reported an alternative procedure to produce hematopoetic stem cells in the same issue of Nature. “They are taking a very indirect approach to reach their goal,” added the first author of this study, Raphael Lis from Weill Cornell Medical College. Lis and Rafii used a novel approach to generate true hematopoetic stem cells in the lab without the need for iPS cells [2].
The team chose endothelial cells that line the blood vessels as their starting material, which they genetically modified by forced overexpression of four transcription factors. The secret ingredient in their recipe involved recreating the natural environment that blood stem cells find comfortable. This “vascular niche” produced growth factors essential for survival and reproduction of the hematopoetic stem cells.
“Our discovery of the vascular niche was the major breakthrough here,” Rafii said. On injecting the cultured hematopoetic cells into irradiated mice, Rafii’s team saw generation of blood and immune cells. “This is the first on its own generation that anybody has ever been able to generate engraftable hematopoietic stem cells from any other source,” said Rafii.
Daley and Bhatia noted that endothelial cells, the starting material in Rafii’s approach, are not easy to obtain or to scale-up. They require the added complication of a donor, since these need to be harvested from adult blood vessels. “So, I think the jury’s still out on how one will proceed with the endothelial cells,” added Bhatia.
Pluripotent cells, on the other hand, can be generated from patients using their blood cells and skin fibroblasts, and the technology to expand them to large scales in cultures is well-established. “From a point of view of production of cells to a clinical scale and doing all analysis you need to do to be sure that the cells are safe, I think pluripotent cells have a real advantage,” Daley said.
And the Winner Is?
It’s tempting to compare the diverse approaches of the two groups to determine an obvious winner; however, as Bhatia pointed out, each method may have its own niche users. “It’s difficult to estimate at this point which is going to be the most robust technology, and I am not sure if they are competing.”
According to Bhatia, one disadvantage of both approaches is the forced overexpression of transcription factors, a step known to increase the risk of inducing tumors. This may be avoided by transiently expressing transcription factors or avoiding this step altogether by substituting with chemicals that perform similar functions. Daley’s team is currently working on expressing genes in a transient manner, just long enough to reprogram the cells.
In the long run, both teams dream of ultimately testing their approach in clinical trials and using the generated hematopoietic stem cells for gene therapy. “This can benefit patients who do not have matched stem cells [from donors],” said Rafii.
Bhatia believes that both these approaches have taken blood cell generation research to the next level. He hopes that building on that foundation, other stem cell researchers can get closer to the clinic. “Everyone can theorize about what might be the best approach, but until you achieve the goal, no one is correct,” Bhatia concluded.
Please enter your username and password below, if you are not yet a member of BioTechniques remember you can register for free.