A glimpse into the cell’s recycling system
Researchers discover how cells recognize cellular components that are ready to be recycled.
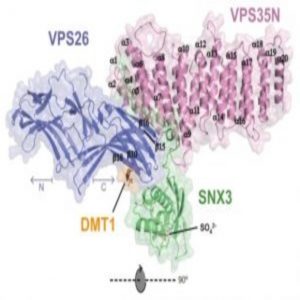
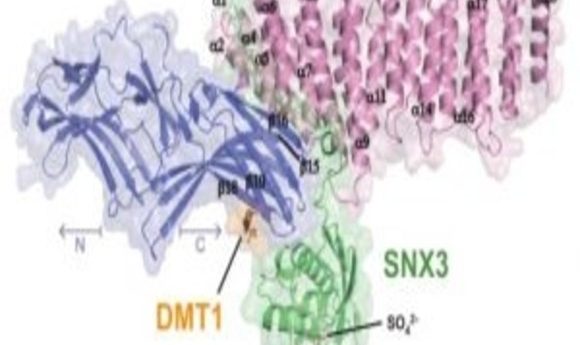
Overall Structure of the VPS26-VPS35N-SNX3-DMT1-II Complex (1).
A cell is like a small city with houses sitting along the membrane. Within this city is a recycling pickup service—a multi-protein complex called retromer—that collects recyclable donations, or transmembrane cargo, from houses participating in the city’s recycling program. Retromer then sends the cargo to the recycling center, or the trans-Golgi network (TGN), for processing.
Although this recycling process runs smoothly in healthy cells, it isn’t clear how retromer goes about its route. What about the structure of retromer allows it to recognize cargo?
Knowing how the recycling system works in a healthy state can provide insights into how it’s disrupted in disease. “For that reason, we started to try and crystalize the remaining structure of retromer, 60% of which was unknown,” said Aitor Hierro, professor at Ikerbasque Basque Foundation for Science and senior author of a new study in Cell describing the structure of retromer and revealing a cooperative process among its subunits during cargo recognition (1).
Retromer consists of two complexes: the vacuolar protein sorting (VPS) heterotrimer, containing VPS26-VPS29-VPS35, which is involved in cargo recognition, and the membrane-targeting complex composed of sorting nexins (SNX).
While trying to figure out the structure of VPS26 bound to VPS35, the team was unable to crystallize the two subunits. They decided to include SNX3 to stabilize the complex. Hierro and colleagues set out to find the missing retromer structure, but in doing so, they came across something surprising.
“When SNX3 interacts with VPS26, there is a conformational change that allows the recognition of the recycling signal,” said Hierro. This conformational change in the structure exposes a binding site that responds to the recycling signals of divalent metal transporter 1 isoform II (DMT1-II), a known membrane cargo that cycles from endosomes to the plasma membrane.
The researchers wanted to test the functional relevance of the structure, so they turned to cell culture. Using HeLa cells where the team removed SNX3 using CRISPR, they saw increased colocalization of DMT1-II with early endosome antigen 1 (EEA1) compared to control cells, signaling a disruption in cargo transport from the endosome to the TGN.
Thus, concomitant interactions of SNX3 with VPS26 and the recycling signals from DMT1-II on the membrane both recruit retromer to the membrane for cargo recognition; the conformational change also allows for cargo selection. To go back to the city metaphor, the houses participating in the recycling program put their recycling on the curb to signal to the trucks, or retromer, that its ready for pickup and transport to the recycling center.
Hierro and colleagues are further investigating how other SXNs may interact with retromer to create another conformation change capable of recognizing more signals. Instead of a single-stream recycling system, retromer recognizes different recycling signals, such as glass versus cardboard. You can imagine SNX3 binding to VPS26 and recognizing DMT1-I as glass recycling. Another SNX may bind with retromer to recognize other cargo that would represent, let’s say, cardboard recycling.