Creating Pinky’s brain: researchers use stem cells to grow mouse embryo with a brain

Do synthetic mice dream of electric sheep? We’re on the way to finding out thanks to a breakthrough in stem cell research.
It’s not going to overthrow mankind from a lab anytime soon, but a stell cell-grown mouse has got the brain. Stem cells are the cornerstone of the body and can differentiate into almost any other type of cell therein. In the week post-fertilization, three types of stem cells develop: one to form the tissues of the body, while one of the extraembryonic stem cell types becomes the placenta, and the other becomes the yolk sac.
By carefully guiding these three types of stem cell, researchers at the University of Cambridge (UK) grew a synthetic mouse embryo with a brain and beating heart, and the beginnings of a full set of organs, without the use of sperm or eggs.
For an embryo to develop, the proto-embryonic tissue and the tissue that will connect the embryo to the mother need to establish contact. The researchers established specific environments and induced particular sets of gene expressions to encourage the stem cells to communicate.
They observed that extraembryonic cells use both chemical signaling and touch to guide their embryonic counterparts to develop into the embryo. Researchers guided this process by assembling cultured stem cells, representing the three sorts of tissue, in specific proportions and environments to coax them into communication. From there, the stem cells self-organized and developed into the formational stages of organs, in addition to the yolk sac in which an embryo gains nutrients during its first weeks.
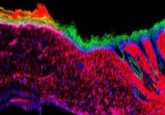
 Novel hydrogel bioink improves 3D-printed biomaterials
Novel hydrogel bioink improves 3D-printed biomaterials
Researchers have overcome the limitations of current bioinks by incorporating microgels, taking us one step closer to bioprinting functioning organs and tissues.
The model even developed a beating heart and the initial stages of an entire brain with evidence of anterior development: a developmental milestone never before achieved in previously created synthetic embryos.
Lead researcher Magdalena Zernicka-Goetz, understandably excited about this breakthrough, enthused: “This opens new possibilities to study the mechanisms of neurodevelopment in an experimental model. In fact, we demonstrate the proof of this principle in the paper by knocking out a gene already known to be essential for formation of the neural tube, precursor of the nervous system, and for brain and eye development. In the absence of this gene, the synthetic embryos show exactly the known defects in brain development as in an animal carrying this mutation. This means we can begin to apply this kind of approach to the many genes with unknown function in brain development.”
A decade-in-the-making, there are multiple implications for this success. For a start, it could help to elucidate the reasons behind the success or failure of a pregnancy, as many pregnancies fail at the stage when the three initial stem cell types interact and communicate with each other to program embryonic development. The results could also guide future medical developments in trauma repair and synthetic organs for transplants. In addition, the development of the anterior part of the brain marks a major milestone for synthetic embryo research.
Though current research is performed using mouse models, the development of human models could signal the potential for organ generation that is otherwise impossible to study in natural embryos. In the UK, a human embryo can only be studied for up to 14 days of development.
Submit to the F1000Research Cell & Molecular Biology Gateway
Take your cell and molecular biology research further with the F1000Research Gateway. Benefit from trusted publishing, open access, and transparent peer review to ensure your findings are shared responsibly and make a lasting impact. Share your discoveries with a global audience while maintaining full control over your work.
Submit your research today at F1000Research Cell & Molecular Biology Gateway.