Finding ‘True North’ for organoid development: how magnetic synthetic cells are guiding the way

 Jacqueline De Lora (left) is a postdoctoral research scholar at the Max Planck Institute for Medical Research (Heidelberg, Germany), whose research looks at 3D cell cultures, specifically organoid cultures, and synthetic cells.
Jacqueline De Lora (left) is a postdoctoral research scholar at the Max Planck Institute for Medical Research (Heidelberg, Germany), whose research looks at 3D cell cultures, specifically organoid cultures, and synthetic cells.
Senior Editor, Tristan Free, chatted with De Lora to find out more about her research developing techniques to improve reproducibility in organoid culture. They also spoke about inclusivity in STEM and the differences between DEI in Europe and America.
What is the focus of your research?
My research focuses on developing organoids. Organoids are miniaturized versions of tissues from the body that are generated using stem cells in different types of environments. The stem cells differentiate and can eventually become different phenotypes of cells that form tissues, for example, heart or bone.
I’m developing techniques to control these different differentiation pathways to improve the reproducibility and the uniformity of organoid culture. I’m using a novel approach focusing on cell-to-cell interactions and cell-to-extracellular matrix interactions in a 3D environment. I use human inducible pluripotent stem cells and I interface them not only with other natural cell types but with a cell that I build myself in the lab, called a synthetic cell. These synthetic cells are built in the lab with techniques from bottom-up synthetic biology and allow me to have precise control over the possible ways that stem cells can interact with synthetic cells.
In our lab, we build these synthetic cells using a droplet-based microfluidics approach, where we generate a water droplet that is surrounded by an oil phase. On the inside of the droplet are small lipid vesicles that chemically bind to the inner surface of the droplet and create a lipid bilayer. Then, we release these droplets out of the oil phase into an aqueous environment, like a biological buffer. We end up with giant unilamellar vesicles (GUVs) that mimic the lipid bilayer of natural cells. We’re able to add complexity to these GUVs by, for example, decorating the surface with different types of proteins, so that we have control over how these synthetic cells behave in different types of culture environments.
One unique way that I am controlling the morphology of the synthetic cell is to use flipped phase behavior. Instead of having a water-based environment on the inside of my synthetic cell, I have an oil-based environment. This oil-based environment enables a suspension of magnetic nanoparticles into the core of the synthetic cell. I call my synthetic cells, ‘synthetic magnetic’ cells, because they have a lipid interface at the surface and oil on the inside that can respond to a magnetic field.
I use E-cadherin proteins, which enable a homotopic interaction between E-cadherin from the stem cell and E-cadherin that I’ve decorated on the surface of my synthetic cell. To integrate these proteins into the synthetic cell surface, we use E-cadherins with a six-histidine tag that can interact with the head group of specially functionalized lipids in the synthetic cell surface. This enables us to chemically attach the protein to the surface of the synthetic magnetic cell. Now, the stem cell and the synthetic cell have a way to communicate directly with one another via protein-protein interactions.
Once I have these interactions established, using a microscope that we built in the lab, I can create a homogeneous magnetic field so that I can control the morphology of the synthetic magnetic cell. When the magnet is inactive, the synthetic magnetic cell exists as a sphere. The morphology then changes once the magnetic field is applied, it elongates and stretches into a more oval configuration. Whenever this happens, there’s a force that is transduced in between the synthetic magnetic cells and natural cells, through the E-cadherin interaction that is linking them together. Now we have a direct mechanotransduction mechanism to apply a force to a stem cell. The idea is that these mechanical interactions on some level control differentiation pathways and by controlling these interactions we can direct the differentiation of stem cells in a culture.
What is the microscope that you built?
We started with an inverted Zeiss microscope and turned it into a Frankenscope. We took out the stage components and we built up a custom platform that has a Helmholtz coil (an electromagnet) on one side. It has iron pole pieces that direct the current flowing through the Helmholtz coil, which creates a magnetic field that goes across the cell culture in one direction. I can make the droplets stretch in the direction of the magnetic field and then relax back as well. By characterizing the dynamics of this morphology shift in the synthetic magnetic cell, we can learn a lot about the local mechanical environment.
How do you monitor cell migration dynamics?
We establish a monolayer of cells that are confined within a chamber and then remove the chamber so that a clear edge and space becomes available to the cells. We then monitor collective cell migration dynamics as the cells move into the space. We use a type of microscopy called traction force microscopy, where the cells are grown on a soft gel that has fluorescent nanoparticles embedded in the gel. This allows you to track the displacement of the nanoparticles as the cells apply tension to the surface of the gel while they’re migrating. From the displacement field, you can also calculate the monolayer stress that is occurring in between the cells.
The thing with monolayer stress microscopy is, it’s a calculation. It’s a derivation of the actual physical forces that you can measure by the displacement of the nanoparticles in the gel, but the monolayer stress itself isn’t directly measured.
For us, the idea was to bring in our synthetic magnetic cell as a ‘spy’ into the monolayer cell culture. The cells are already generating forces against each other all the time, but now we can deform or change the morphology of our synthetic magnetic cell to a degree that we’ve calibrated. When you get a handle on how the protein-protein and force transduction mechanics are behaving, you gain control over the collective cell migration and maybe even gain a direct measurement of the monolayer stresses.
Could you explain more about the extracellular matrix and organoids?
It’s well understood that the extracellular environment is fundamental to forming organoids. Often, people use a commercially available protein cocktail that is undefined and has batch-to-batch differences, like Matrigel. This means that if two people in separate labs are trying to perform the same protocol, they would very likely end up with a completely different outcome.
So, we need a better extracellular matrix material because we want to be able to control how these interactions occur. We’re borrowing a technique from optogenetics, which enables us to use light to control the presentation of RGD ligands (a bioactive ligand) to integrins on the stem cells. LOV2, a well-known optogenetic protein, is conformationally unfolded in blue light illumination, and we can genetically modify it so that it has RGDs in the core. When the protein is in the dark, it’s conformationally folded and the RGD sequences are not available, so integrin cannot bind onto it. This is a light-driven way to present RGD to integrins of the natural stem cells in a space- and time-controlled manner.
The technique that we use to exploit this technology is to encapsulate our stem cells into droplets that contain these light-sensitive proteins. We create these 3D environments and use precise optical set-ups so that we can shine blue light and control when and where the RGD sequences are available for the stem cells to bind to. The timing of these interactions, combined with the physical interactions with other cells can all be used to control the differentiation switch of stem cells in a mechanobiological sense.
Our dream here is that we will be able to set up an optogenetic system, in combination with our synthetic magnetic cells, which can be programmed to produce a specific type of tissue that is exactly the same each time. So, in essence you would be able put your starting cells into this theoretical automation machine, tell it to produce a model of heart tissue through a user-friendly interface and then it will generate a 3D model of that tissue for you.
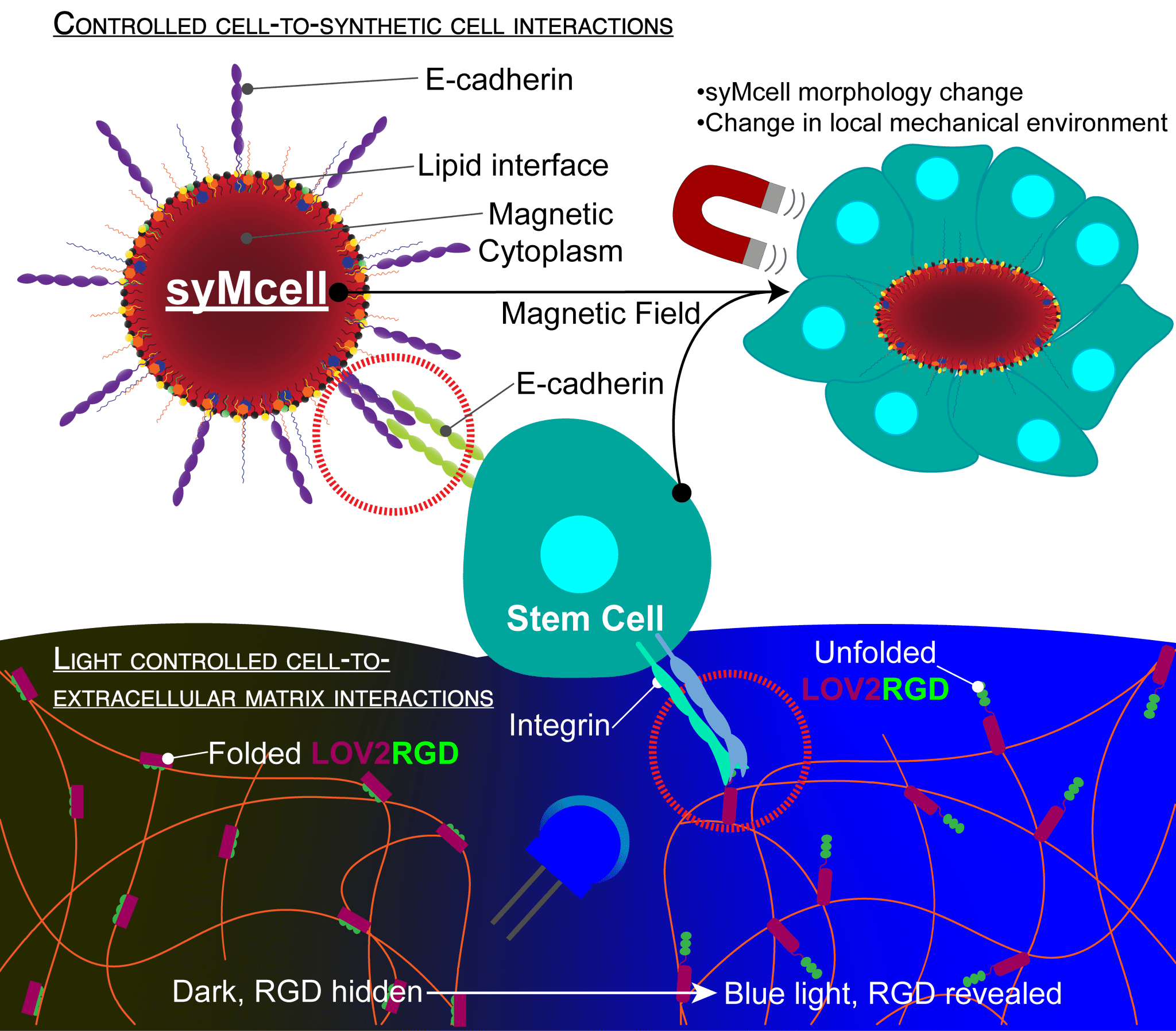
Figure 1: An illustration of Jacqueline’s synthetic magnetic cells and light-controlled ECM, and the control that can be exerted on stem cells using these new technologies.
What are some of the challenges of generating tissues with these processes?
The challenge lies in the calibration. For example, we need a good understanding of the dynamics of the deformation of the synthetic magnetic cells as well as the optogenetics tools and how the natural cells respond to these manipulated interactions. We have to do our own quality control to make sure that we understand how all of these combinatorial parameters are behaving. We need to understand the force generation profiles between the synthetic cells and natural cells, the mechanical response of the cells as RGDs are revealed within their environment, and how that could be affecting the differentiation of stem cells, or not.
What further developments would you like to see in organoid technology?
It’s important to consider statistics within organoid cultures. What really needs to happen is a transition into batch bioreactor types of cultures, where you have large volumes and huge numbers of organoids within one population. Along with this, you need to start to consider the development of other types of analysis techniques, like high-throughput imaging approaches or large-particle flow cytometers, that allow you to benefit from flow-based analysis when you have millions of organoids in a culture. This way, you have an answer to if the variable you’re looking at is making an impactful difference in the organoid development pipeline.
Can you tell us a little bit about your journey through academia from New Mexico across to Germany?
I have a BS in Biology and a BA in Chemistry from the University of New Mexico (NM, USA). I didn’t know what I wanted to do when I graduated but some of my professors were probing me saying, why don’t you go into research? I applied to an NIH-funded post-baccalaureate research education program (PREP), which gives research experience and support for the graduate school application process. It’s a year-long program and I gained admission to my graduate program at the School of Medicine at the University of New Mexico. That’s when I started my PhD studies. What was cool about that program is that they encourage interdisciplinarity. Even though I was at the School of Medicine, I was still allowed to travel across campus to the engineering department and join a lab in the Center for Biomedical Engineering, which was where I started in the world of 3D-cell culture.
I had my first exposure to synthetic cells during a short postdoc at the University of Mexico, in the Chemical and Biological Engineering Department. That’s when I met my current mentor Joachim Spatz (Max Planck Institute, Heidelberg, Germany). I ended up moving to Germany in February 2020, right as the pandemic set in, and, naturally, there were some drawbacks to my lab work and integrating into society. But eventually, both happened.
Where do you see issues of inclusivity in STEM?
For me, inclusion comes from the individual with support from the institution, whereas diversity is more at a high level. As an individual, I only have so much control over the diversity of my environment, but I have a direct handle on inclusivity. The issue is then inclusivity within an institution may suffer when there is not a leveled understanding from all individuals as to why inclusivity is needed in the first place. So, implementing trainings and a culture where all backgrounds and viewpoints are encouraged is essential.
Are there differences in how DEI is handled in Europe and America?
The core of the problem is that race and ethnicity data is not collected in the European Union (EU), it’s even against the law to request demographic information from individuals in countries like Germany and France, so they’re lacking the data on how effective their DEI programs are. For example, I sort of lost my identity a little bit when I first moved to Germany because I came from a US-based minority-serving institution where my minority status as a female Hispanic scientist was documented and championed. But there’s no reporting for this data in Germany, so within the Max Planck Institute for example, I don’t know the status of any other scientists with my phenotype. A perfect example of the appropriate use of these types of data in the EU is in gender equality where institutions have the ability to leverage information and work to minimize disparities. Similarly, there needs to be a way to collect demographic data in a very safe and secure way so that individuals are confident that supplying sensitive information will empower institutions to leverage it in the proper manner and know if their DEI programs are actually making an impact.
Are there any upcoming Hispanic scientists, organizations or resources that you’d like to shout out?
I’m very far removed, spatially and otherwise, from any Hispanic community. However, I believe that in 2021, the EMBL started an Equality, Diversity and Inclusion program, and they have a lot of amazing resources for people with a Hispanic background based in Europe looking to integrate into the community.
My shout-out is to Christina Termini, who recently started her lab and is becoming a Twitter sensation! It’s inspiring to see her forge her path and I’ve learned from her willingness to transparently share her triumphs and her failures and how she’s overcoming them.