New organoid biobank proves a useful tool for head and neck cancers
A new organoid biobank has demonstrated the ability to validate biomarkers, test personalized treatment strategies and study novel drugs for head and neck cancers (HNCs).
A recent collaboration between researchers at the University Medical Center Utrecht and the Hubrecht Institute (both Utrecht, The Netherlands) has produced a biobank of over 100 organoids for the study of HNCs. Subsequent tests of these organoids have validated their clinical relevance and generated insights into existing treatment patterns that could lead to more personalized and effective treatment regimes for people with HNCs.
Existing treatments for HNCs include various combinations of surgical resection, radiotherapy and chemotherapy; however, these treatment regimens can have serious side effects that prevent patients completing the treatment course. What’s more, due to the significant genetic heterogeneity between the tumors of different patients, the selection of the ideal treatment strategy is challenging. As a result, completion of the assigned regimen provides no guarantee for patients, with 60% of HNC patients relapsing post treatment.
To address this issue, oncologists need better models that represent tumor heterogeneity to test treatment strategies against. In an attempt to address this, the team established a biobank of 110 models, 65 of which were tumor models, from HNC patient tissue.
 Introducing organoid intelligence: human brain cells that power biocomputers
Introducing organoid intelligence: human brain cells that power biocomputers
I know you’ve heard of artificial intelligence, but what about organoid intelligence? Researchers from multiple disciplines are working together to create biocomputers powered by 3D cultures of human brain cells.
To validate the utility of these models, DNA sequencing was used to establish that the DNA alterations found in HNCs were replicated in the organoid models and immunohistochemistry confirmed retention of the same histological features. Once this had been established, the organoids were challenged with several treatments and monitored for cell death. This revealed that, for both chemoradiotherapy – with cisplatin (Fig. 1) and carboplatin – and adjuvant radiotherapy, the organoids responded similarly to the responses of the patients from whose tissue they were generated from, indicating their clinical predictive value.
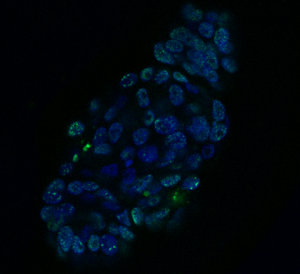
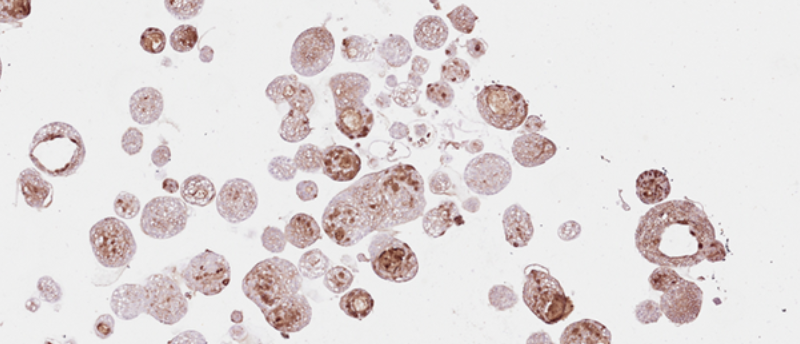
Figure 1. Organoid derived from a HNC patient, treated with the drug cisplatin. Cell nuclei are visible in blue and DNA damage in green. Credit: Organoid group, copyright Hubrecht Institute.
Of particular note, the team identified that tumor organoids were desensitized to radiotherapy when they had previously been treated with the chemotherapy drug cetuximab. Expounding on the significance of this discovery, corresponding author Else Driehuis (Hubrecht Institute) noted that, “this is surprising, because the combination of this drug and radiotherapy is given to some HNC patients in the clinic today. In patients, it’s difficult to distinguish the individual contributions of the drug and the radiation therapy to the overall effect of this combination treatment, but in the organoids we can pull that apart. Our results fit with recent published data that show that survival of patients treated with cetuximab and radiotherapy is worse compared to treatment with radiotherapy alone.”
The team also identified a novel PRMT5 inhibitor, which is currently in clinical trials for different cancers, that could serve as a new treatment option for a subset of HNC patients. Co-first author Rosemary Millen (Hubrecht Institute) explained how they, “sequenced the DNA of the organoids, to investigate the relationship between specific genetic mutations and the response to treatments. By doing so, we found that tumors with loss of the gene CDKN2A were responsive to treatment with this novel drug. It would be very interesting to see whether this effect is also found in patients, especially since this mutation is present in over 50% of [head and neck squamous cell carcinoma] HNSCC cases.”
Further investigations using CRISPR technologies to introduce specific mutations allowed the team to test the predictive power of tumor biomarkers.
From this single study, primarily designed to validate the utility of the biobank and to test its functionality, multiple clinically relevant discoveries have been made that require further testing. First, the use of cetuximab may be best implemented following radiotherapy; second, a PRMT5 inhibitor shows efficacy against HNC tumors with CDKN2A knocked out; and third, that these models can be used as an initial test of personalized treatment strategies for patients. Now complete, it will be fascinating to see what findings emerge from this biobank in the future.