Mapping the road to more sophisticated organoid systems
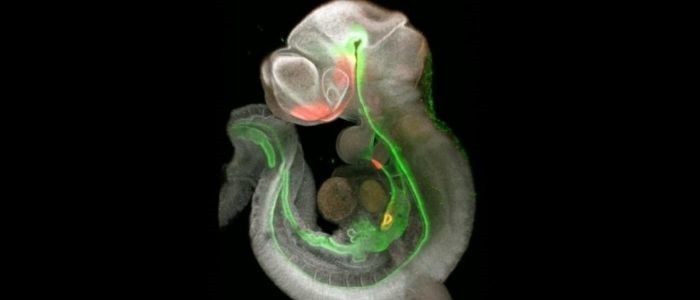
Two papers recently published in Nature Communications signal a landmark in the development of more complex and representative organoid systems.
The papers come as the result of a collaboration launched in 2019 between the Center for Stem Cell & Organoid Medicine (CuSTOM) at the Cincinnati Children’s Hospital Medical Center (OH, USA) and RIKEN (Tokyo, Japan). The collaboration aims to drive forward innovations in organoid technology.
The initial paper set out to establish the signals in the foregut of the embryo – a proto-organ that later develops into the trachea, esophagus, stomach, liver, pancreas, gallbladder and the caudal portion of the duodenum – that result in the differentiation of the structure into its subsequent organs.
To investigate these signals, the team employed single-cell RNA sequencing to obtain information about the transcriptomes of the embryonic mouse foregut. Using this information, they identified a disparate set of cells capable of initiating and distributing the signals responsible for stimulating the growth of the organs that succeed the foregut.
Focusing on the timeframe in which organ induction occurs in the mice – embryonic days 8.5-9.5 – the team looked at the molecular signaling activity to produce a detailed map of the foregut’s development in mice, indicating how and why the organs form at specific locations.
Vital to the formation of these organs is the development of specialized mesenchyme cells that become the smooth muscle and fibroblast tissue of the organs. The signaling roadmap identified SHH-driven signals as essential to the diversification of the splanchnic mesoderm into the specific mesenchymal cells required for different organs, while WNT-driven signals were also essential for the organ’s formation.
 Modeling a heart attack: researchers develop human cardiac organoids
Modeling a heart attack: researchers develop human cardiac organoids
Researchers have developed human cardiac organoids in order to model myocardial infarction and drug cardiotoxicity.
Extrapolating this information, the team then turned to human cells to see if their differentiation could be controlled. Describing the outcome, senior author Aaron Zorn (CuSTOM) stated that, “we then used this information from the mouse to differentiate the equivalent human tissue in the lab. This is important because up until now all of the liver, lung, stomach and esophagus organoids that we make mostly lack these mesenchyme cell types.”
This roadmap, in addition to increased control over the development of human tissues, will allow the team to build on the work of Takanori Takebe (CuSTOM), who assisted with the study and was the first to successfully develop a connected three organoid system in 2019. However, Takebe’s system lacked some of the cell types needed for complete organ function, something that the team may be able to correct with the new road map.
The second paper digs deeper into these signaling interactions, focusing wholly on the development of the trachea. Using genetically modified mice, the team was able to establish which signals were paramount to the correct formation of the trachea.
Zorn highlighted the importance of this second paper, explaining that the paper, “helps explain what happens when birth defects like esophageal atresia, tracheoesophageal fistula, and tracheomalacia occur. This work also opens the door to one day generating esophagus and trachea tissue for tissue replacement.”
The potential of this work is clear. With the signaling road map and more in-depth information on the development of specific organs, the production of more synergistic and representative organoid systems may become possible. These organoid systems can then be used to model developmental diseases and birth defects, whilst also being able to better test novel medications.
If techniques can be developed to further upscale the growth of these organ systems, there is also the possibility that labs will be able to generate tissue to be used in regenerative medicine to repair or even replace damaged and dying organs.