Novel patient-derived parathyroid organoid developed
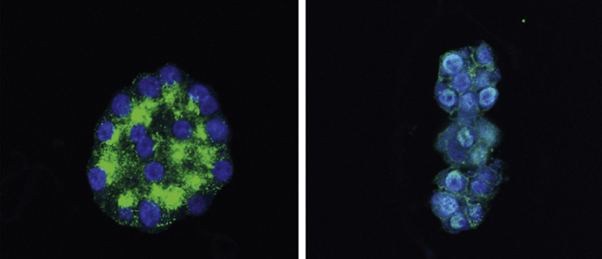
Researchers have developed the first patient-derived parathyroid organoid (PTO), which accurately mimics parathyroid structure and function and could improve in vitro research and drug development.
Researchers at the University Medical Center Groningen (Netherlands) led by Robert Coppes and Schelto Kruijff have developed the first ever patient-derived parathyroid organoid (PTO). The authors believe that their research will enable more accurate in vitro testing for drug development and opens the door to a potential hypoparathyroidism treatment. The current study demonstrates that PTOs developed from parathyroid stem cells can accurately model human parathyroid tissue structure and functionality.
Parathyroid diseases are frequently caused by abnormal parathyroid hormone secretion, which leads to impaired calcium homeostasis. Whilst researchers have previously noted the mechanism involved in primary hyperparathyroidism, there are many parathyroid diseases with unknown pathophysiologies.
In this study, the team obtained human benign hyperplastic parathyroid tissue from two surgical patients and isolated parathyroid stem cells by observing self renewal in single cells. They subsequently cultured these cells to recapitulate parathyroid differentiation to form differentiated patient-derived parathyroid organoids (dPTOs) before testing to demonstrate their comparability to the original human tissue.
 Superorganism identified that can ‘leap’ from tooth to tooth
Superorganism identified that can ‘leap’ from tooth to tooth
A recent study finds evidence of multi-cellular cross-kingdom superstructures that can move from tooth to tooth, causing more extensive tooth decay.
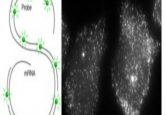
To conduct these tests and assess the parity of these dPTOs to the original tissue, the team used RNA bulk sequencing and immunofluorescence analysis. These analyses showed that the organoids have a comparable gene expression profile for key parathyroid gene markers and overall displayed limited differences to the original tissue. The researchers also found that the differences in gene expression between the parathyroid tissues from patients one and two were mirrored in the dPTOs.
Healthy parathyroid tissue responds to high extracellular calcium or the typical hyperparathyroidism treatment, cinacalcet, by reducing parathyroid hormone secretion. Although the models lacked the original parathyroid microenvironment, such as its vasculature and the variations in extracellular signal concentration, the study demonstrated that the dPTOs react to both an increase in extracellular calcium and cinacalcet in a comparable manner to the parathyroid tissues they were derived from. Together with the comparable gene expression, this finding suggests the PTOs could be a useful model for assessing the parathyroid as they were phenotypically and functionally similar.
The authors believe that their PTO offers a novel option for parathyroid research. Senior co-author Kruijff said, “we are the first group in the world that was able to isolate parathyroid stem cells and maintain these cells in our lab as organoids for an extended period of time.” He added, “these organoids mimic the patient condition, are able to produce hormone, express specific markers, and show comparable reactions to drugs.”
The researchers’ next plan is to transplant these organoids into rats with hypoparathyroidism to observe the PTOs’ function in vivo. Kruijff suggested that in the future the technique could be applied to treat disease, saying “Also, this technique could be used to try to culture healthy parathyroid gland organoids in order to treat patients with hypoparathyroidism.”
Submit to the F1000Research Cell & Molecular Biology Gateway
Take your cell and molecular biology research further with the F1000Research Gateway. Benefit from trusted publishing, open access, and transparent peer review to ensure your findings are shared responsibly and make a lasting impact. Share your discoveries with a global audience while maintaining full control over your work.
Submit your research today at F1000Research Cell & Molecular Biology Gateway.