RalA: the culprit for mitochondrial dysfunction in obesity
Researchers have highlighted that eating a high-fat diet dismantles mitochondria via the activation of a single molecule termed RalA.
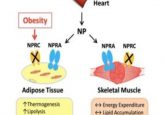
Obesity is characterized by fatty tissue expanding and undergoing several functional changes, including inflammation, apoptosis and fibrosis. Mitochondria play a key role in metabolic regulation, with a delicate balance of fission and fusion controlling their effects. Previous research has suggested associations between mitochondrial dysfunction, reduced energy expenditure and insulin resistance; however, not much was known about the mechanisms underlying these links.
In a recent study, scientists from the University of California San Diego (CA, USA) fed mice a high-fat diet for 8–12 weeks and closely studied the effect it had on the metabolic functioning of the rodent’s white adipocyte cells.
The team observed through electron microscopy that white adipocytes are impacted by a high-fat diet, with their mitochondria becoming fragmented, resulting in reduced oxidative capacity. The team also observed that a fatty diet increased the expression and activity of the small GTPase termed RalA.
“Caloric overload from overeating can lead to weight gain and also triggers a metabolic cascade that reduces energy burning, making obesity even worse. The gene we identified is a critical part of that transition from healthy weight to obesity,” commented lead author of the study Alan Saltiel (University of California San Diego, CA, USA).
 New genes discovered in the fight against obesity
New genes discovered in the fight against obesity
Through studies on C. elegans, 14 new genes have been discovered that could be used to develop treatments for obesity.
When the team deleted RalA in the white adipocytes of mice fed a high-fat diet, rodents were both protected from mitochondrial fragmentation and obesity, even when they ate the same high-fat diet as their counterparts who did gain weight.
The findings demonstrate that chronic activation of RalA plays a key role in repressing energy expenditure in obese adipose tissue by shifting the balance of mitochondrial dynamics toward excessive fission, contributing to weight gain and metabolic dysfunction. Furthermore, the team discovered some proteins in the studied mice, which were affected by RalA, are analogous to human proteins and are associated with obesity and insulin resistance.
“By understanding this mechanism, we’re one step closer to developing targeted therapies that could address weight gain and associated metabolic dysfunctions by increasing fat burning,” reported Saltiel.
In terms of next steps, the team wishes to investigate the mechanisms underlying this process in more detail with the hope of one day using their findings to help treat or even prevent obesity by targeting the RalA pathway.