The Golgi gold standard: identifying and producing robust T-cells for cancer immunotherapy

Researchers have identified that the status of the T-cell Golgi apparatus is a key indicator of performance in cancer immunotherapy approaches.
A group of researchers working at the Medical University of South Carolina Hollings Cancer Center (SC, USA) led by Shikhar Mehrotra, have recently identified the Golgi apparatus as a determinant of success in certain cancer immunotherapies. Pre-treatment to increase resistance to Golgi stress and cell sorting to select cells with high Golgi content both resulted in more effective therapies in mice. It is hoped that applying this knowledge could refine existing cancer immunotherapy approaches and aid in designing more effective recombinant T-cells.
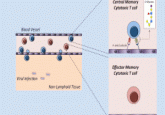
Adoptive transfer of tumor-reactive T-cells is a cancer immunotherapy approach in which T-cells attuned to the destruction of cancer cells, are administered to a patient. These cells can originate from a separate donor or the patient themselves. In the latter instance, cells can be genetically modified to express chimeric antigen receptors (CARs) to target cancer cells more effectively, an approach known as CAR-T cell therapy.
However, a major drawback of this strategy is the lack of longevity following infusion. The immunosuppressive nature of the tumor microenvironment (TME) exerts oxidative stress on both native and infused T-cells, ‘exhausting them’. Mehrotra’s lab explores ways to minimize this exhaustion and boost the performance of T-cells in the TME.
The Golgi apparatus plays a vital role in cells, modifying and sorting of thousands of proteins before their delivery to intra- and extracellular locations, and is known to exhibit a stress response that enables the organelle to modify its structure to respond to the needs of a specific environment. However, the specific impact of the TME on the Golgi apparatus is poorly understood.
Identifying this organelle as a potential target to improve the performance of T-cells in the TME, the team set out to investigate the stress response of the Golgi to this environment and identify potential strategies to fortify it.
“Helper” T-cells pack more of a punch against tumors than first thought
CD4+ T-cells, primarily considered to play a supportive role in the anti-tumor immune response, have been seen to actively kill cancer cells in a mouse model of melanoma.
To do this, the researchers pre-treated murine T-cells with hydrogen sulfide (H2S), a gaseous signaling molecule shown to minimize Glogi stress and stabilize the immune response in previous mammal studies, which they hypothesized would reduce stress in the antitumor T-cells and extend the duration of their activity in the tumor. This was achieved via the overexpression of the enzyme cystathionine β-synthase (CBS), in the H2S synthetic pathway.
The team identified that H2S modifies the cysteine residues of the protein PRDX4 in the Golgi apparatus, protecting it from oxidative stress and preventing its fragmentation. Focussing on the Golgi, the researchers used fluorescence-activated cell sorting cell-sorting to select T-cells in the top and bottom 30% for Golgi content.
In studies performed on a human lymphoma xenograft mouse model, human CD19 CAR-T cells sorted for high Golgi content or for higher levels of H2S via CBS overexpression, significantly prolonged survival compared to CAR-T cells sorted for low Golgi content. These results, taken together indicate that the function of the Golgi apparatus is key to resisting the immunosuppressive effects of the TME.
These pre-clinical findings present some exciting avenues for future research. First, the amount of Golgi apparatus could be used to select only the most effective T-cells for adoptive transfer. “Essentially, if you just use the Golgi apparatus as a simple marker, if T-cells have a lot of Golgi versus less, the ones that have more Golgi are much more robust at killing tumor cells and controlling tumors,” elaborated Nathaniel Oberholtzer, lead author of this study.
Further, the upregulation of H2S could be used to develop more robust and long-lasting CAR-T cell therapies via the incorporation of CBS or related enzymes into the CAR-T construct during genetic modification.