Traitors of the aging brain
Refer a colleague
Astrocytes are trusty helpers of the brain, but a new study reveals that they may betray all that they once held dear in our golden years.
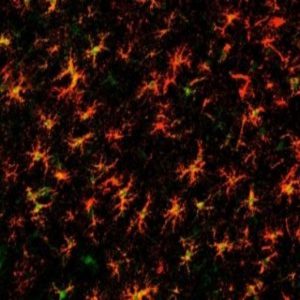
Astrocytes don’t often attract publicity, despite their importance in brain function. The star-shaped cells are involved in caretaking and defending the brain, and they are critical for neuronal synaptic signalling. During development, astrocytes sculpt the nervous system circuits, ensuring correct neuronal pairings through stimulation or elimination of synapses. Synapse numbers decrease in the aging brain in a process associated with cognitive decline, and Nicola Allen’s group at the Salk Institute wondered whether astrocytes play a role in this process.
“We know a lot of things in the young brain that can regulate synapses, so we wanted to know, do they get switched on in aging?” said Allen. The team found that some inflammatory pathways used by astrocytes in eliminating synapses during development switched on again in the aging brain. These findings, published in Cell Reports, suggest that astrocytes are active players in age-related cognitive decline and that targeting astrocytic pathways could protect synapses in the aging brain.
Allen’s team analyzed the transcriptomes of the astrocytes using a ribotag technique to specifically isolate astrocyte RNA from the different regions of adult (4 month old) and aging (2 year old) mouse brains. Astrocytes from different brain regions are distinct in their gene expression profiles, so the team used cluster analyses to identify what genes changed in each individual region with age.
The researchers were surprised by how little the astrocytes changed over time; many core genes involved in astrocytic maintenance of homeostasis and synaptic transmission remained unaltered. However, there was one exception. “The major rate-limiting enzyme in the cholesterol synthesis pathway is down-regulated in astrocytes in all brain regions by age,” said Allen. Astrocytes are a major source of cholesterol, which is important for synaptic vesicle formation and is reduced in the aging brain.
Elderly astrocytes in almost all brain regions displayed a mildly inflammatory genetic profile. “There is strong upregulation in two of the members of the complement cascade, C3 and C4, suggesting that in the absence of any disease, astrocytes are starting to produce an environment that allows synapse elimination,” said Allen. Some of the inflammatory genes upregulated in age also associate with neurodegenerative diseases, although diseased astrocytes show a much greater increase in inflammatory gene expression.
“Region-specific and age-related changes in astrocyte reactivity possibly contribute to archetypal neurodegenerative conditions such as Alzheimer’s and Parkinson’s diseases,” said Rickie Patani from University College London, who was not involved in the study.
Allen is curious about the link between aging astrocytes’ gene expression profiles and those seen in neurodegenerative diseases, leading her to wonder if a pre-pathological state could be identified and used as a disease predictor.
Allen and her team posted the transcriptome profiles online, where they are freely available. “Studies on aging in a rodent system costs a lot of money to do, so it seemed pointless for us to do that and then not give the data to other people,” said Allen. “No one should have to repeat this!”
The long process of studying aging in animals is causing Allen’s group to consider using new techniques to culture astrocytes in vitro, and to perhaps develop a system that induces astrocytes to recapitulate the traitorous gene profiles of aging seen in vivo.
Please enter your username and password below, if you are not yet a member of BioTechniques remember you can register for free.





