Two become one
Scientists have pinpointed a protein that gametes use to fuse with one another during sexual reproduction—and it’s the same protein that viruses use to infiltrate cells. What does this mean for treating some of the world’s worst diseases?
Haploid gametes patch their plasma membranes together and combine genetic information during fertilization to form a new individual. This process is vital to many organisms on Earth, but little is known about the molecular mechanisms behind the cell–cell fusion.
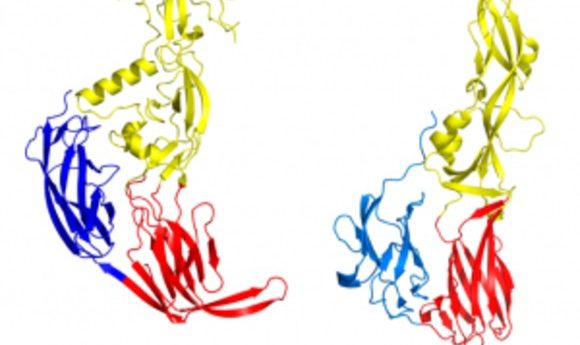
In a recent issue of Cell, researchers described the molecular function of HAP2, a key protein in gamete fusion. They also showed that HAP2 is identical to the protein that viruses such as Zika and Dengue use to infect human cells.
“I find that amazing that the protein that sperm in many organisms use to penetrate the egg is the same one that viruses use to penetrate and infect our cells,” said co-lead investigator William Snell, now of the University of Maryland. “It’s a new target for science to focus on to figure out ways to block reproduction of organisms that cause much harm and misery for human beings.”
Snell studies Chlamydomonas, a single-celled blue-green algae that often reproduces asexually in pond water. When conditions are bad, two different mating types of the species each form haploid gametes, which fuse together to form a spore-like zygote. Snell’s team previously discovered that HAP2, a cell-surface protein with one end anchored in the membrane and the other sticking out into the environment, is critical for fertilization in the alga.
The researchers later learned that the same protein had already been discovered in Arabidopsis and lily plants, where it was thought to function in adhesion of the gametes. In the new study, Snell’s group showed that HAP2 was instead necessary for the actual merger of the membranes.
Using computer modeling, Snell and his collaborators predicted that the structure of HAP2 was similar to that of class II viral fusion proteins. One part of the protein in particular, the fusion loop, looked critical to membrane fusion, so Snell’s team investigated its function with 2 experiments: in the first, they mutated a single amino acid in the loop; in the second, they generated an antibody against a 20 amino-acid section of the loop. In both cases, gamete fusion was blocked in Chlamydomonas.
Snell then teamed up with Felix Rey at the Pasteur Institute to determine the structure of HAP2 using X-ray crystallography, which showed definitively that it was a class II fusion protein.
The resulting new model for gamete fusion is identical to viral fusion: HAP2 has its “feet” in the membrane of one gamete. When it approaches the membrane of its partner, the protein is somehow triggered to insert its “head” (the fusion loop) into the partner’s cell membrane. With HAP2 now bridging the two cells, the protein undergoes a dramatic conformational change, essentially bending over at the “waist,” to pull the two membranes against each other forcefully enough that they merge to become one.
HAP2 appeared early in evolution and is present in organisms from protozoa (including Plasmodium, which causes malaria) to multicellular animals like insects. “The more exciting part potentially—though it’s all speculation right now—is I’m guessing that antibodies against the same part of the Plasmodium protein will be able to be used as a transmission-blocking vaccine against malaria,” said Snell.