Why do we have sex cells?
Why is the use of a germ line for reproduction almost exclusive to the animal kingdom? New research now overturns a previously accepted hypothesis explaining why animals developed sex cells.
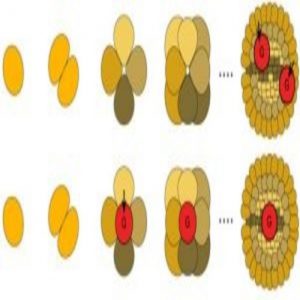
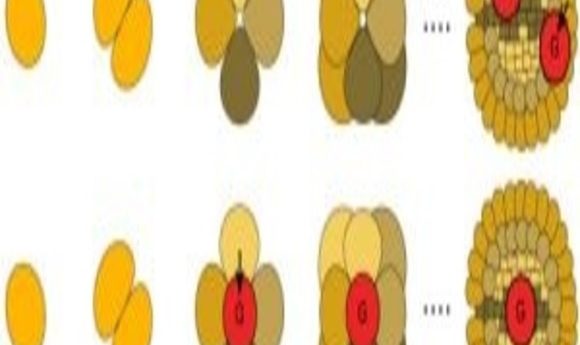
Plants and basal animal species develop gametes later in development (top), whereas most animals have early gamete development (bottom) (1).
It pays to plan ahead. Within the animal kingdom, developing embryos are already considering their progeny, constructing the germ cells that will produce the gametes used in procreation. The exceptions to this are basal animal species such as coral and sponges. While scientists have some ideas on the matter, it is not clear why reproductive behaviour in animals has diverged from that of other life forms. Now, research recently published in PLoS Biology flips a previously accepted hypothesis on its head by pointing a finger at mitochondria.
“Previously, the selfish cell conflict was used to understand why we have a germ line,” said corresponding author Nick Lane from University College London. This relates to multicellular competition, with a strict distinction between germ-line and somatic cells that prevents these cells from competing with each other.
“However, there are issues with this theory with limited conditions feasibly creating these selfish cells,” said Lane. “We decided to model mitochondrias’ influence. All cells have a population of mitochondria, so we were thinking in terms of how you deal with mitochondrial DNA during reproduction. People had touched on this before but never in a systematic way.”
Using mathematical modelling, the team tested two scenarios: where germ cells did not immediately develop and where a germ line was present from the start. The models shed light on a mechanism used to protect the quality of mitochondria. If cells repeatedly divide to form adult tissue before gametogenesis, then the mitochondria could quickly accumulate a ton of genetic mutations. Producing germ cells early on prevents these mutations from passing to the next generation and rendering a fitness cost to the species.
Most animals have a high error rate for mitochondrial gene replication. To prevent harmful errors from creeping into progeny genes, there is a restricted burst of cell division during early development. This creates a varied population of gametes with no need for any further division, reducing the risk of accumulating harmful mutations. Mutations can continue to accumulate in sperm, but this problem is avoided through mitochondria being inherited maternally.
In plants and basal animals, the researchers found a lower error rate in mitochondrial gene replication. Therefore, these species can worry less about accumulating these errors, so gametes are produced later in development, eliminating the germ line’s need.
To understand why there is a difference in replication errors, Lane’s team explored metabolism. During the Cambrian Period, there was a shift from filter feeding to predation, which raised aerobic activity and, therefore, the need for mitochondria. Increased numbers of mitochondria in turn increased the error rate of replication.
Going forward, “We want to finesse our model, looking at random segregation during development and the effect of different mutation rates in tissues and aging,” said Lane.

