Business of BioTechniques 2024

Welcome to the 2024 Business of BioTechniques blog provided by Zyme Communications. This feature highlights the latest news and industry collaborations, from products and services to important upcoming events and key information in the biotech industry.
To keep up with industry news, don’t miss our monthly updates!
December update
Drug discovery & development
Sphere Fluidics announces Cyto-Mine Chroma to accelerate and streamline workflows across expanded applications in biotherapeutic

Sphere Fluidics’ Cyto-Mine Chroma
Sphere Fluidics (Cambridge, UK), a leading provider of innovative droplet-based microfluidics solutions for single-cell analysis and isolation, shared details of Cyto-Mine® Chroma, the second generation of its flagship Cyto-Mine platform. This new platform will offer enhanced capabilities, including multiplexing and greater assay flexibility, to further maximize the efficiency and precision of single-cell functional analysis workflows.
Alleo and Ubiquigent enter AI-driven strategic partnership to accelerate DUB-focused drug discovery
Ubiquigent (Dundee, UK), a drug discovery and development company harnessing novel deubiquitinase (DUB) modulators as new therapeutics for areas of high unmet medical need, announced a strategic partnership with Alleo Labs Corp., a pioneer in using AI to develop new therapeutics for neurological diseases. The partnership will combine Alleo’s AI-based approach to developing novel therapeutics with Ubiquigent’s platform and expertise in the field of DUB focused drug discovery.
VALANX Biotech and Fina Biosolutions introduce ClickCRM for rapid conjugate vaccine development
VALANX Biotech (Vienna, Austria), a biotech company developing novel technology for site-specific protein conjugation in drug and diagnostics discovery, and Fina Biosolutions (MD, USA), experts in conjugate vaccine development and conjugate chemistry, announced the signing of a joint IP and licensing agreement. The companies will launch ClickCRM™, a ready-to-conjugate version of CRM197, an antigenic carrier protein used for making conjugate vaccines. The new product enables the rapid, high-efficiency conjugation of carrier proteins to polysaccharide antigens for the generation of conjugate vaccines.
Software and laboratory automation
Sapio Sciences enhances bioanalysis LIMS and ELN with advanced immunogenicity capabilities
Sapio Sciences Sapio Sciences (Baltimore, USA), the science-aware™ lab informatics platform, announced the addition of new immunogenicity bioanalysis features to its industry-leading lab informatics platform. These new capabilities in the Sapio LIMS (Laboratory Information Management System) and Sapio ELN (Electronic Lab Notebook) solutions streamline testing workflows for detecting anti-drug antibodies and neutralizing antibodies. Kevin Cramer, Founder and CEO of Sapio Sciences, commented: “The integration of immunogenicity bioanalysis into our LIMS and ELN will enable scientists to conduct highly regulated and complex bioanalytical studies with greater confidence, efficiency, and accuracy.”
Optibrium demonstrates superior molecular docking method for small molecules and macrocycles
Optibrium (Cambridge, UK), a leading developer of software and AI solutions for molecular design announced the publication of a peer-reviewed study in the Journal of Computer-Aided Molecular Design, ‘Structure-Based Pose Prediction: Non-cognate Docking Extended to Macrocylic Ligands’. The paper demonstrates that Surflex-Dock can accurately predict the binding conformation and orientation (pose) of unknown (non-cognate) ligands, including macrocycles. Using a data set of ~1000 ligands, the study compared different molecular docking methods to predict the non-cognate binding of non-macrocyclic and macrocyclic ligands.
H.E.L Group introduces iso-BTC e to advance semiconductor technologies

H.E.L’s iso-BTC e
H.E.L Group (London, UK), a global developer and manufacturer of innovative laboratory tools for process optimization, safety, and scale-up, introduced iso-BTC e, a benchtop isothermal calorimeter for the precise measurement of heat dissipation in semiconductors, either individually or in situ on a printed circuit board. With this, manufacturers can utilize precise heat power data to develop effective thermal management strategies and designs.
Cell & gene
Bio-Rad launches high-precision Vericheck ddPCR Empty-Full Capsid Kit to advance development of safe and effective gene therapies
Bio-Rad’s Vericheck ddPCR Empty-Full Capsid Kit
Bio-Rad Laboratories (CA, USA), a global leader in life science research and clinical diagnostics products, launched Vericheck ddPCR™ Empty-Full Capsid Kit for the determination of capsid titer, genome titer, and percentage of full capsids in purified or unpurified (crude lysate) AAV samples. Using Bio-Rad’s Droplet Digital™ PCR technology, the new Vericheck ddPCR Empty-Full Capsid Kit analyzes minimal amounts of either crude lysate or purified samples to deliver robust, reproducible data for reliable assessment of AAV vector quality.
FUJIFILM Irvine Scientific adds key products to its life sciences portfolio from FUJIFILM Wako Chemicals U.S.A.
FUJIFILM Irvine Scientific (CA, USA), a global leader in the innovation and manufacture of cell culture solutions for the Life Science and Medical markets, has further expanded its product portfolio and enhanced services through the alignment of specific commercial operations with its sister company, FUJIFILM Wako Chemicals. Effective October 1, 2024, products from three divisions within the FUJIFILM Wako Chemicals organization — Lab Automation, Lab Chemicals, LAL Group — will be promoted directly from FUJIFILM Irvine Scientific to customers in the United States and Europe.
Cellular Origins, Fresenius Kabi sign development agreement for scalable automation of cell and gene therapy manufacturing
Cellular Origins (Cambridge, UK), and Fresenius Kabi, an operating company of Fresenius (Frankfurt, Germany), have signed a development agreement that leverages each company’s expertise in cell and gene therapies (CGTs). The agreement is designed with the goal of digitally and physically integrating Fresenius Kabi’s suite of cell therapy processing technologies within Cellular Origins’ CGT robotic manufacturing platform Constellation™. The aim of this work is to assist cell therapy developers to manufacture their therapies at scale using their preferred processing tools.
Axol Bioscience acquires Phenocell to advance human disease models
Axol Bioscience (Cambridge and Edinburgh, UK), a leading induced pluripotent stem cell (iPSC) technology provider for drug discovery, has fully acquired Phenocell SAS (Grasse, France), a pioneer in iPSC-based products and bioassays for skin and retinal disorders. The acquisition extends Axol’s portfolio of iPSC-derived cell models, adding skin and human retinal iPSC-derived cell lines to its existing neuroscience, pain and touch, and cardiovascular products and services.
Finance and funding
Nuclera closes $75 million USD financing
Nuclera (Cambridge, UK), the biotechnology company accelerating protein expression and purification workflows through its benchtop protein system, has completed a USD $75 million financing round (circa £57 million). The fundraise was led by Elevage Medical Technologies, backed by Patient Square Capital, and joined by British Patient Capital, the largest domestic investor in UK venture and venture growth opportunities, Cambridge Innovation Capital, Jonathan Milner, GK Goh, M&G Catalyst, E Ink Holdings, Michael D. McCreary, Uni Power Group, and Verve Ventures. Taylor Wessing advised Nuclera on the transaction. The investment will enable the continued commercialization of Nuclera’s eProtein Discovery™ benchtop system, particularly in the US and across Europe.
Shift Bioscience raises $16M to advance cell simulation AI platform
Shift Bioscience (Cambridge, UK), is a biotech company using generative AI models to understand how activation of different genes can reverse the aging process. This research is a fundamental step forward in designing more effective drugs to treat age-related diseases. Today the company announced that it has raised $16M (£12.5M) in Seed funding, led by BGF, with existing investors F-Prime Capital, Kindred Capital, and Jonathan Milner participating. The investment will be used for the continued development of Shift Bioscience’s artificial intelligence cell simulation platform, for the identification of genes that can safely rejuvenate cells to combat the effects of age-related illnesses.
Antiverse raises £3.5M ($4.6M) to advance generative AI antibody design platform
Antiverse (Cardiff, Wales), a techbio company designing antibodies for challenging targets, has added £3.5M ($4.6M) to its seed funding. The investment will facilitate the Company’s growth, including appointments to strengthen the team and continued development of its antibody design programmes, many in partnership with pharmaceutical companies. The latest investment round was led by i&i Biotech Fund I and Kadmos Capital, with additional investment from existing investors InnoSpark Ventures, UKI2S (managed by Future Planet Capital), Tensor Ventures, and AngelHub. This brings the total equity financing raised to £7.2M ($10.1M).
Clinical Trials
Calluna Pharma successfully completes Phase 1 clinical study of CAL101, a first-in-class therapeutic for fibrotic and fibro-inflammatory indications
Calluna Pharma (Oslo, Norway), a clinical stage biotechnology company pioneering first-in-class antibodies to treat inflammatory and fibrotic diseases, announced the completion of Phase 1 clinical study for CAL101, Calluna’s lead product candidate. The study demonstrated a favorable safety, pharmacokinetic and immunogenicity profile for the mAb. CAL101 is a first-in-class mAb that targets S100A4, a damage-associated molecular pattern protein implicated in serious and life-threatening diseases, such as idiopathic pulmonary fibrosis and systemic sclerosis.
People
Calluna Pharma appoints Mark Gaffney as Chief Executive Officer and Mark Altmeyer as Independent Chair of the Board
Mosaic Therapeutics appoints Dr Barry Davies as CSO
Metrion Biosciences appoints Lee Patterson as CEO
September update
Drug discovery & development
Newcells Biotech launches Lung Ciliary Beat Frequency assay to enhance safety and efficacy profiling in preclinical drug development
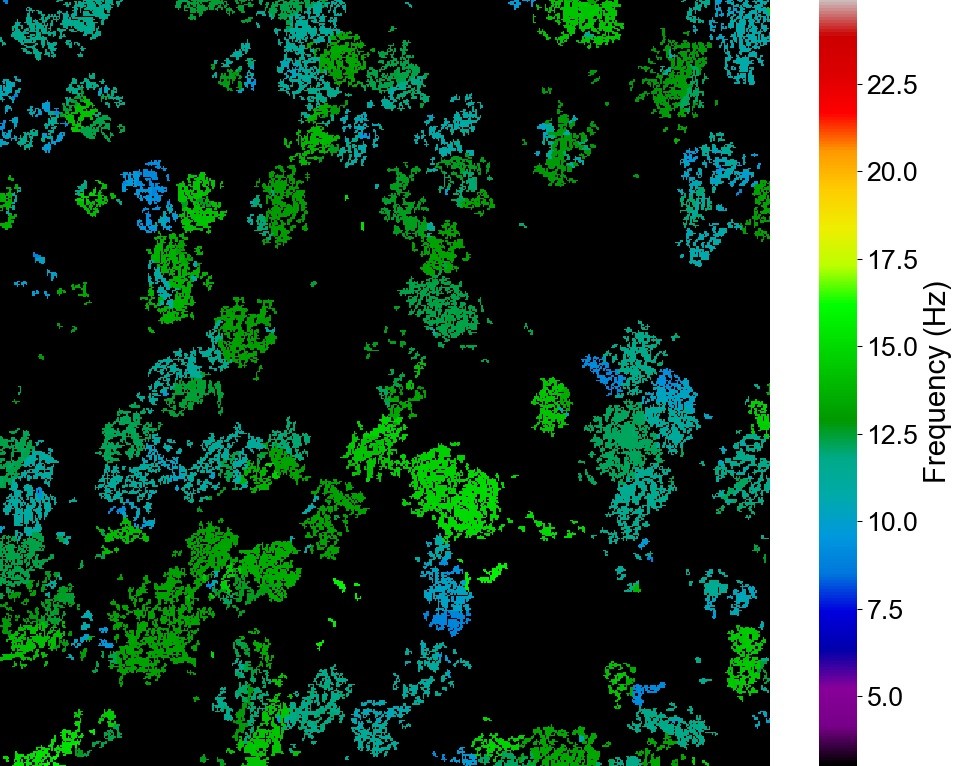
Heat map from CiliaBeat software demonstrating distribution of actively beating ciliated cells with color-coded beat frequency
Newcells Biotech (Newcastle, UK), a leading drug discovery partner specializing in the development of in vitro models and bespoke assay services to improve the prediction of in vivo human outcomes, launched its proprietary CiliaBeat software and application in a Lung Ciliary Beat Frequency Analysis assay. The cutting-edge software enables researchers to assess the effect of drugs and chemicals on the function of the ciliated cells within the lung small airways. The technology has been extensively validated using Newcells’ physiologically relevant and established small airway epithelial cell model, demonstrating high sensitivity and predictiveness for safety and efficacy in preclinical drug development.
Launching alongside CiliaBeat and designed to harness the full capabilities of the software, Newcells’ Lung Ciliary Beat Frequency Analysis assay expands the company’s services portfolio. The new, functionally validated assay generates reliable and predictive in vitro data on the activity of the beating cilia within the airways. It allows the assessment of dosing on mucus clearance, cilia function and evaluation of drug efficacy in reversing disease-like conditions.
Kadans Science Partner commences work on new purpose-built wet lab facilities at Merlin Place, North Cambridge
Kadans Science Partner (Cambridge, UK), a leading investor in the development of ecosystems and real estate with a dedicated focus on knowledge-intensive sectors, announced that construction work has started at Merlin Place, North Cambridge. The 139,000 sq. ft development, expected to complete in 2026, will feature six stories of state-of-the-art lab and office space, allowing for full flexibility for a single- or multi-tenant split. It has been designed to become the ‘Gateway Building for the Cambridge North Cluster’, and a specialist hub for health, life science and technology companies of different sizes.
Software and laboratory automation
Sapio Sciences advances the world’s first AI-powered lab assistant
Sapio Sciences (MD, USA), the science-aware™ lab informatics platform, announced significant enhancements to Sapio ELaiN, the pioneering AI-powered lab assistant that helps scientists streamline processes and work more efficiently.
Sapio ELaiN, now generally available after a successful beta launch in November 2023, combines the power of advanced large language models to support scientists and streamline time-consuming, repetitive or tedious lab tasks and assist in interacting with scientific and analytical agents, enabling researchers to focus on high-value activities and accelerate scientific discovery. As part of the Sapio Lab Informatics Platform, Sapio ELaiN works seamlessly with Sapio LIMS, Sapio ELN, and Sapio Jarvis to simplify life in the lab, aiding scientists and lab operations in efficiently configuring processes and workflows, improving day-to-day operations, and enhancing data analysis and reporting.
Recently, Sapio Sciences announced that leading informatics providers and consultants Zifo, CREO, EPAM and Astrix, have joined the Sapio Sciences Partner Program with a shared commitment to fostering productivity and accelerating science.
CrestOptics’ CICERO spinning disk confocal system receives Top 10 Best Microscopy Innovation Award from Microscopy Today 
CrestOptics (Rome, Italy), a manufacturer of high-end microscopy solutions and advanced systems for fluorescence microscopy and diagnostic applications, announced that its CICERO spinning disk confocal system has been named a ‘Top 10 Best Microscopy Innovation’ by Microscopy Today, an industry-leading microscopy publication from the Microscopy Society of America. Launched in April 2023, CICERO was developed as a compact and cost-effective solution to improve access to high-end imaging for the wider scientific community.
The CICERO system is an all-in-one solution for widefield and confocal imaging, expanding CrestOptics’ portfolio to enable accessible fluorescence imaging within one compact, budget-friendly, yet high-performing solution. The spinning disk confocal instrument has been designed and engineered to easily integrate into existing and custom workflows, with maximum configuration flexibility, to support a seamless and user-friendly transition across a broad range of applications in basic and applied research.
Cell & gene
MIP Discovery rebrands as Tozaro as it completes transition to supporting cell and gene therapy viral vector analytics and purification
MIP Discovery (Bedfordshire, UK) announced the company’s rebrand to Tozaro, completing its transition to becoming a technology innovator in the cell and gene therapy (CGT) bioprocessing sector. The rebrand comes at a time of significant growth for the company, following the refocusing of its mission to improve downstream processing within the CGT sector by helping to transform the development and manufacture of viral vectors, alongside its successful £7M Series A funding round in February. The company’s updated brand identity fully supports this shift in focus, underpinning its commitment to enable innovation within CGT bioprocessing with its smart polymer technology.
CRISPR
ERS Genomics and Medicines Discovery Catapult sign CRISPR/Cas9 license agreement
ERS Genomics (ERS; Dublin, Ireland), the CRISPR licensing company, and Medicines Discovery Catapult (‘MDC’), an independent, not-for-profit drug discovery innovation center, have signed a non-exclusive commercial-use CRISPR license agreement. The partnership combines ERS’s CRISPR/Cas9 patent portfolio with MDC’s world-class expertise and technology.
MDC and its partners will benefit from access to ERS’ entire CRISPR/Cas9 portfolio – the foundational CRISPR/Cas9 intellectual property held by Emmanuelle Charpentier. This will extend MDC’s ability to provide breakthrough products and services that help de-risk drug discovery, drive sector productivity and attract investment.
Diagnostics
Bio-Rad outlines its advances leveraging Droplet Digital PCR in biopharma and translational research sectors
Bio-Rad Laboratories (CA, USA), a global leader in life science research and clinical diagnostic products, provided an overview of advances supporting the company’s overall corporate strategy to improve science and healthcare in the year to date. Centered on Bio-Rad’s Droplet Digital™ PCR solutions, achievements include strategic research collaborations with leading oncology institutions and health networks to enhance clinical monitoring of cancer patients, as well as expansion of the company’s product portfolio to support the increased use of Droplet Digital PCR across the biopharma and translational markets.
“We are delighted to report such significant advancements in the first half of the year, which exemplify Bio-Rad’s commitment to delivering innovative technologies and solutions that will drive the life sciences industry forward,” said Morgan Norris, Senior Vice President of Marketing, Bio-Rad Laboratories. “Bio-Rad’s collaborations with leading oncology institutions and health networks will be instrumental in establishing Droplet Digital PCR as a foundational technology for molecular residual disease monitoring, supporting our overarching strategy to advance scientific research and healthcare worldwide.”
Flow cytometry
Bio-Rad launches Annexin V StarBright Conjugates for apoptosis detection via flow cytometry
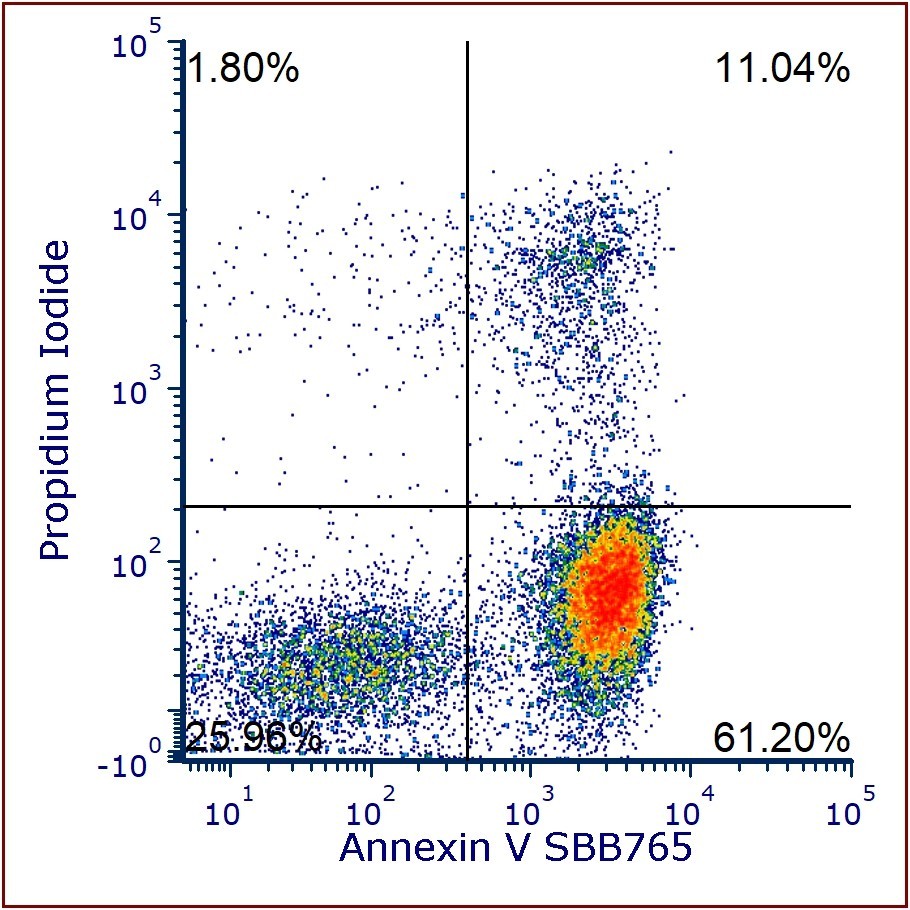
Bio-Rad’s Annexin V StarBright Conjugates
Bio-Rad Laboratories (CA, USA), announced the launch of annexin V conjugated to eight StarBright™ Dyes: SBUV400, SBUV795, SBV440, SBV515, SBV790, SBB675, SBB765, SBY800. The new Annexin V StarBright conjugates support detection of early apoptotic cells by flow cytometry, offering an increased range of fluorophore options. Bio-Rad’s range of annexin V conjugates provides researchers with a variety of choices, especially important when including apoptosis detection in multicolor immunophenotyping panels in both conventional and full spectrum flow cytometry.
Annexin V StarBright conjugates enable full utilization of all the common laser lines found in flow cytometers such that common viability dye and fluorescent protein emission wavelengths can be avoided. This, combined with the narrow excitation and emission of StarBright Dyes, reduces spillover and spreading to provide high-resolution data.
Finance and funding
PhoreMost extends series B financing to US$50 million to advance high-value oncology and inflammation degrader programs
PhoreMost (Cambridge, UK), a leading UK biopharmaceutical company unlocking the next generation of drug targets, has added US$12 million to its Series B financing, bringing the total raised to over US$50 million. The additional investment was led by Parkwalk Advisors, the largest growth EIS fund manager, with participation from existing investors BGF, Jonathan Milner, Amadeus Capital and Astellas Venture Management, and will support the progression of PhoreMost’s pipeline of novel degrader assets.
PhoreMost is focused on leveraging its SITESEEKER® target ID platform to enable novel ligase discovery. Since the closure of its previous Series B financing in 2021, the company has expanded drug discovery operations and advanced multiple next-generation degrader programs through proof-of-concept studies following these discoveries.
NanoSyrinx closes £10 million financing and appoints Edwin Moses as Chairman

Dr. Edwin Moses, Chairman, NanoSyrinx
NanoSyrinx (Coventry, UK), a synthetic biology company developing nanosyringes as a novel platform for targeted intracellular delivery of biologic therapeutics, has closed a c. £10 million (c. US$13 million) financing round. The funding will support further advancement of the company’s technology platform to accelerate the development of biologic therapeutics against a pipeline of previously ‘undruggable’ intracellular targets.
The round was co-led by BGF, Octopus Ventures and M Ventures, with support from existing investors, IQ Capital and Meltwind. Global medicines company Eli Lilly and Company also participated. The funding will be used to drive further development of NanoSyrinx’s technology platform, to realize its full potential in advancing intracellular medicine. Alongside the financing, Edwin Moses has joined as NanoSyrinx’s Chairman, taking over from Stephen Taylor who has led the board since the company’s inception. Edwin is a renowned entrepreneur and C-level executive in the life sciences industry, bringing over 30 years of board-level experience from over 20 life science companies.
People
Broken String Biosciences appoints Steve Becker as Chief Commercial Officer
Sphere Fluidics appoints Alex Dickinson as Non-Executive Director
Enterprise Therapeutics appoints Annabella Amatulli as Head of Regulatory Affairs
Metrion Biosciences appoints Chris Mathes as Chief Commercial Officer
July update
This month’s Business of BioTechniques blog highlights several new products, as well as technological advancements in AI and laboratory informatics. We also detail manufacturing and bioprocess developments, clinical trial news of a novel cystic fibrosis therapy, funding to advance the treatment of Parkinson’s disease, and feature several key company appointments.
Drug discovery & development
Bio-Rad launches ddSEQ™ Single-Cell 3′ RNA-Seq Kit for single-cell gene expression
Bio-Rad Laboratories (Hercules, USA), a global leader in life science research and clinical diagnostics products, launched the ddSEQ™Single-Cell 3′ RNA-Seq Kit and complementary Omnition v1.1 analysis software for single-cell transcriptome and gene expression research.
Designed to be run on Bio-Rad’s droplet-based single-cell isolation system, the ddSEQ Cell Isolator, the ddSEQ Single-Cell 3′ RNA-Seq Kit delivers high-quality single-cell 3′ RNA-Seq libraries in a fast, efficient, and affordable workflow, allowing researchers to easily conduct single-cell gene expression and regulation analyses. The subsequent QC, analysis, and reporting of data generated from the ddSEQ Single-Cell 3′ RNA-Seq Kit is enabled by the accompanying Omnition v1.1 Analysis Software, a robust pipeline analysis tool.
Sphere Fluidics awarded Gold accreditation by Investors in People
 Sphere Fluidics (Cambridge, UK), a leading provider of innovative microfluidics-based solutions for single-cell analysis and isolation has received the ‘We invest in people’ Gold accreditation, a prestigious award presented by Investors in People (IIP). This accolade is in recognition of the Company’s commitment to excellence in people management and continuous improvement in workplace practices.
Sphere Fluidics (Cambridge, UK), a leading provider of innovative microfluidics-based solutions for single-cell analysis and isolation has received the ‘We invest in people’ Gold accreditation, a prestigious award presented by Investors in People (IIP). This accolade is in recognition of the Company’s commitment to excellence in people management and continuous improvement in workplace practices.
The IIP framework is a globally-recognized standard for people management, defining what it takes to lead, support, and manage people effectively to achieve sustainable results. Presentation of the Gold accreditation signifies how Sphere Fluidics has not only met but surpassed the high standards set by IIP when assessing the Company’s practices, policies, and overall approach to investment in people.
Software and laboratory automation
Sapio Sciences introduces Sapio GMP LIMS offering unmatched flexibility for unique manufacturing processes
Sapio Sciences (Baltimore, USA), the science-aware™ lab informatics platform, announced the Sapio GMP (Good Manufacturing Practice) LIMS for laboratories that require unparalleled flexibility to meet manufacturing compliance standards. The new Sapio GMP LIMS solution addresses industry applications in biotechnology, pharmaceutical, clinical research and diagnostics, food and beverage, chemical, and environmental testing.
Built on Sapio Sciences’ industry-leading lab informatics platform, the new GMP solution includes a quality control laboratory information management system, environmental monitoring programs, and stability management. The solution addresses applicable regulatory requirements, data integrity, and security to ensure global compliance and provides test and result management, documentation, robust audit trails, and electronic signatures.
The Sapio Sciences Partner Program launched in July, providing a growing partner ecosystem of technology vendors, services companies, and resellers with the tools, training, support, and marketing services to deliver solutions that empower scientists.
Bio-Rad launches ChemiDoc Go Imaging System for highly sensitive benchtop gel and western blot imaging
Bio-Rad Laboratories (Hercules, USA) announced the launch of the ChemiDoc™ Go Imaging System, the latest addition to its portfolio of ChemiDoc Imaging Systems. The system offers rapid, reliable, and highly sensitive gel and western blot imaging on a benchtop scale.
The ChemiDoc Go Imaging System uses complementary metal oxide semiconductor digital imaging to capture gel and western blot images with the same high sensitivity as larger instruments. The compact, user-friendly imager supports advanced chemiluminescence as well as fluorescence detection using StarBright™ Dyes, and its three sample trays provide the versatility with a wide range of applications, including nucleic acid and protein quantitation through various detection methods.
Closed Loop Medicine selected to join NVIDIA Inception
Closed Loop Medicine (London, UK), a leading TechBio company developing combination prescription drug-plus-software precision therapy products that deliver personalized dose optimization to treat the individual, not just the disease, has joined the NVIDIA Inception program. Led by world-leading Artificial Intelligence Computing company, NVIDIA, the accelerator program is designed to nurture startups and early-stage companies revolutionizing industries with technological advancements. The program will support the Company as it drives development of its expanding portfolio of ML-driven predictive dosing products and entry into broader therapeutic areas with high unmet clinical need.
Optibrium demonstrates accelerated lead optimization in complex agrochemical development
Optibrium (Cambridge, UK), a leading developer of software and AI solutions for molecular design announced the publication of a peer-reviewed study in the Journal of Computer-Aided Molecular Design, ‘From UK-2A to florylpicoxamid: Active learning to identify a mimic of a macrocyclic natural product’. The paper demonstrates the successful application of the QuanSA (Quantitative Surface-field Analysis) method, part of Optibrium’s BioPharmics platform for 3D molecular design, to accelerate the lead optimization of a complex macrocyclic natural product during agrochemical development. By significantly reducing the number of synthetic steps required during optimization, the study supports the commercial viability of complex macrocyclic compounds.
Cell & gene
Asimov introduces its AI-driven 4th generation CHO Edge System with increased titer guarantee
Asimov (Boston, USA), the synthetic biology company advancing the design and manufacture of therapeutics, Asimov, the synthetic biology company advancing the design and manufacture of therapeutics, launched its fourth generation CHO Edge System. With an increased typical titer range of 5-11 g/L across modalities before any upstream process optimization, the new system has been developed to optimize expression across a breadth of biologic architectures and increase the likelihood of high titer cell lines.
The 4th generation CHO Edge System updates expression vector architectures, genetic parts selection, and process methodologies. In addition, the new system incorporates a suite of AI models to predict signal peptide cleavage, RNA splicing, and upstream process optimization. Customers can access these advanced capabilities by licensing the CHO Edge System or as part of Asimov’s Cell Line Development Service
Cellular Origins acquires ACTIA Platform IP to enhance automated cell therapy manufacturing
Cellular Origins (Cambridge, UK) a TTP Company, focused on enabling scalable, cost-effective, and efficient manufacture of cell and gene therapies (CGTs), announced the acquisition of the ACTIA (Autologous Cell Therapy Industrial Automation) Platform IP, developed by Geoff Hodge whilst CEO of SOTIO Biotech US. The ACTIA Platform complements Cellular Origins’ existing approach and will accelerate and expand R&D efforts to further develop the Company’s robotic solution for automated cell therapy manufacturing, Constellation™.
Launched in May 2023, Constellation combines advanced automation robotics with aseptic fluid-handling technologies in a highly flexible and scalable system. Uniquely, the platform physically and digitally integrates with a wide range of existing bioprocessing equipment, enabling users to adopt its use without requiring significant changes to either the workflow or equipment, alleviating the risks and time that come with process redevelopment.
Finance and funding
Lario Therapeutics awarded $6M grant from The Michael J. Fox Foundation for Parkinson’s research
Lario Therapeutics (Edinburgh, UK), a biopharmaceutical company developing first-in-class precision medicines for epileptic and neurological disorders, has been awarded a $6 million USD grant from The Michael J. Fox Foundation for Parkinson’s Research. The program is in collaboration with the Oxford Parkinson’s Disease Centre, which will also be delivering key science as part of the grant.
The grant will be used to fund Lario’s preclinical program investigating selective CaV2.3 calcium channel inhibition as a novel and disease-modifying approach for treatment of Parkinson’s disease. There is extensive literature linking calcium channels to pathology of the disease, and it has been demonstrated in preclinical experimental studies that the deletion of CaV2.3 can have a protective effect against Parkinson’s disease progression. Lario is partnering with Professor Richard Wade-Martins and the Oxford Parkinson’s Disease Centre for the study, for the evaluation of Lario compounds in state-of-the-art patient-derived stem cell models of Parkinson’s disease.
Clinical Trials
Enterprise Therapeutics doses first person with cystic fibrosis in phase 2 trial for novel therapy ETD001
Enterprise Therapeutics (Brighton, UK), a biopharmaceutical company dedicated to the discovery and development of novel therapies to improve the lives of those suffering from respiratory disease, announced dosing of the first person with cystic fibrosis (pwCF) in its Phase 2a trial of ETD001.
ETD001, a low molecular weight compound with first-in-class potential, targets the epithelial sodium channel (ENaC) in the airway epithelium to increase the hydration and clearance of mucus. The Phase 2a trial aims to deliver clinical proof-of-concept and to assess the safety profile of ETD001 in the 10% of pwCF with the highest unmet medical need. The study will be performed at sites located in UK, Germany, France and Italy and will assess lung function (FEV1) in pwCF who are either ineligible for or are not receiving CFTR modulators. Previously, Enterprise Therapeutics published a preclinical profile of ETD001 in the Journal of Cystic Fibrosis.
People
Mogrify appoints Dr Jonathan Appleby as Chief Scientific Officer
Optibrium Appoints James Halle as Chief Commercial Officer
Newcells Biotech appoints Emile Shaffu as Director of Sales and strengthens executive and US team with key appointments
Enterprise Therapeutics appoints Dr Renu Gupta as Chief Medical Officer and Dr Janet Hammond as Non-Executive Director
H.E.L Group Appoints Professor Yih-Shing Duh as Chief Scientific Advisor
CN Bio appoints Lydia Seymour as VP People and Culture to further strengthen leadership team
Cellular Origins Appoints Geoffrey Hodge as Non-Executive Director
June update
This month’s Business of BioTechniques blog highlights continued growth in the drug discovery and precision medicine sectors, including product developments, academic collaborations, and software and AI solutions. The appointment of specialist distributors and key senior positions has been made at several UK-based companies to further advance the industry.
Drug discovery & development
PhoreMost introduces GlueSEEKER platform for discovery of molecular glue degraders
PhoreMost (Cambridge, UK), a leading UK biopharmaceutical company unlocking the next generation of drug targets, announced the introduction of GlueSEEKER™. The new phenotypic screening platform expands the Company’s capabilities in this emerging therapeutic modality to support the systematic discovery and development of novel molecular glue degraders for targeted protein degradation.
The new GlueSEEKER platform has broad-ranging applications and is able to identify induced degradation events for almost any nominated neosubstrate and ligase pair, expanding the scope for this important modality and providing the Company and its partners with opportunities for novel therapeutic pipeline development. In June, PhoreMost achieved a second milestone in its multi-project target discovery collaboration with Boehringer Ingelheim.

Sphere Fluidics Cyto-CellectPLUS
Sphere Fluidics launches Cyto-CellectPLUS to accelerate cell line development workflows
Sphere Fluidics (Cambridge, UK), a leading provider of innovative microfluidics-based solutions for single-cell analysis and isolation, announced the launch of Cyto-Cellect®PLUS. In conjunction with the Company’s Cyto-Mine® platform, the new assay provides a streamlined method to measure antibody production in single cells by rapidly detecting secreted human IgG, enabling the identification and selection of cells with the highest productivity for more efficient cell line development.
PlaqueTec and the Babraham Institute collaborate on early phase target discovery and development
PlaqueTec (Cambridge, UK), a company identifying endotype-specific biomarkers to advance precision medicine for coronary artery disease, announced the successful completion of a collaboration to evaluate lead compounds predicted to bind a pro-inflammatory protein discovered by PlaqueTec in pilot studies. The project was funded by the UKRI-BBSRC Campus Innovation Award, via the Babraham Research Campus Collaboration Fund in October 2023.

Nuclera’s eProtein Discovery™ system
Nuclera eProtein Discovery system installed at leading academic institutes
Nuclera (Cambridge, UK), the biotechnology company accelerating protein expression and purification workflows through its benchtop protein system, has successfully completed 11 installs of its eProtein Discovery™ system, including at leading academic institutes. The system has been placed in academic institutions including University College London, University of Cambridge, University of Manchester, The Flanders Institute for Biotechnology in Belgium, and the CRUK Cambridge Institute.
Gyros Protein Technologies introduces Gyrolab Generic Anti-AAV Kit to support gene therapy development
Gyros Protein Technologies (Uppsala, Sweden), a pioneer in automated nanoliter-scale immunoassays and leading provider of peptide synthesizers, announced the introduction of its Gyrolab® Generic Anti-Adeno Associated Virus (AAV) Kit. The new ready-to-use kit facilitates the qualitative assessment of pre-existing binding antibodies against AAV vectors, enabling screening in pre-clinical and clinical settings. The kit supports identification of pre-existing immunity that may interfere with the efficacy of AAV-based gene therapy delivery.
DefiniGEN and Atelerix collaborate to ship in vitro cell models internationally without the need for freezing or cryopreservation
DefiniGEN and Atelerix (Cambridge and Newcastle, UK), have successfully shipped in vitro liver models from the UK to a top tier pharma customer in the US. The agreement between the companies combines DefiniGEN’s mechanistically relevant iPSC hepatocytes (Opti-Heps) with Atelerix’s hydrogel preservation technology, which prevents loss of function and enables even sensitive samples to remain stable at ambient temperatures for up to two weeks.
SolasCure Selected for Innovate UK Global Incubator Programme in Houston
SolasCure (Cambridge, UK), a biotechnology company developing a novel treatment to transform chronic wound care, has been selected for Innovate UK’s Global Incubator Program in Houston, US. The program, which is focused on commercializing innovative solutions for unmet healthcare needs, is delivered in partnership with the Texas Medical Centre, the largest medical complex globally. The program will support the future entry of Aurase Wound Gel, SolasCure’s first investigational product for the treatment of millions of patients with chronic wounds worldwide, into the US market.
Secarna Pharmaceuticals and Orbit Discovery enter collaboration to discover and develop peptide-conjugated targeted antisense oligonucleotide therapeutics
Secarna Pharmaceuticals (Martinsried, Germany) a leading independent European antisense drug discovery and development company, and Orbit Discovery (Oxford, UK), a leader in the discovery of therapeutic peptide hits, have collaborated to discover and develop peptide-conjugated targeted antisense oligonucleotide (ASO) therapeutics. The collaboration will leverage Orbit’s expertise and bead-based peptide display engine for the identification, screening, and selection of cyclic peptides specific to a wide range of disease targets, to be paired with Secarna’s ASO molecules.
MediMab Biotherapeutics opens bespoke laboratory facilities, created by Kadans Science Partner, at Abingdon Science Park, UK
MediMab Biotherapeutics (Oxford, UK), a subsidiary of MediMabBio, Inc., a South Korea-based biotechnology firm, has opened a new state-of-the-art facility, developed by Kadans Science Partner, at Sovereign House, Abingdon Science Park, UK. The purpose-built building, including state-of-the-art CL2 wet labs, will support the company’s growth and accelerated development of immuno-oncology therapeutics. They are moving from The BioEscalator in Oxford where they have previously operated from. MediMab Biotherapeutics’ discovery platform uses advances in systems biology combined with sophisticated immunological solutions to identify significantly improved first-in-class immuno-oncology drugs that can actively target a range of advanced and solid tumors.
Cell & gene
Cellular Origins and Cell and Gene Therapy Catapult collaborate to demonstrate universal automation of CGT manufacturing
Cellular Origins, a TTP Company, (Cambridge, UK), focused on enabling scalable, cost-effective, and efficient manufacture of cell and gene therapies (CGTs), and the Cell and Gene Therapy Catapult (CGT Catapult), an independent innovation and technology organization specializing in the advancement of the cell and gene therapy industry, have collaborated to demonstrate universal automation of CGT manufacturing.
Finance and funding
Nuclera awarded £1.14M Innovate UK funding for development of eProtein discovery
Nuclera (Cambridge, UK), has been awarded two highly competitive Innovate UK grants totaling £1.14M. The funding, made up of a £790k flexible, agile, scalable, and sustainable technologies grant awarded in collaboration with DeepMirror, and a £350k Engineering Biology grant, to further develop the platform, including evaluations by Dr Konstantinos Beis, an independent expert from Imperial College London, and Dr Andrew Quigley from Diamond Light Source.
ArgusEye closes €2.8 million funding to accelerate bioprocessing
ArgusEye (Stockholm, Sweden), a provider of innovative sensor solutions for real-time monitoring of biological systems, closed its funding, raising €2.8 million EUR (~£2.4 million GBP) bringing the total raised by the Company to €3.45 million EUR (~£3 million GPB). The funding was led by Voima Ventures, a Nordic early-stage investor that specializes in supporting science-based solutions, and co-led by existing investor Eir Ventures, a Scandinavian life science venture capital fund, following its investment into ArgusEye’s seed funding.
Software and laboratory automation
Closed Loop Medicine and Pharmanovia commence OptiZest study for their first precision medicine combination therapeutic
Closed Loop Medicine (London, UK), a leading TechBio company developing combination prescription drug plus software therapy products that deliver personalized dose optimization, and Pharmanovia (Basildon, UK), a global pharmaceutical company that commercializes novel medicines and revitalizes, extends and expands the lifecycle of established medicines, initiated their OptiZest study, ID: NCT06372470, and first patient recruitment. OptiZest will investigate whether patients with hypertension can improve blood pressure control at home while receiving a personalized dose of Zestril (lisinopril).
Optibrium partners with FMC Corporation to transform agrochemical discovery
Optibrium (Cambridge, UK), a leading developer of software and AI solutions for drug discovery, has signed a license agreement for its AI-powered discovery platform, Cerella™, with FMC Corporation (FMC), a global leader in crop protection. Under the agreement, FMC will implement Cerella to enhance its agrochemical discovery programs and accelerate development of its pipeline.
H.E.L Group extends sales and support with appointment of specialist distributors
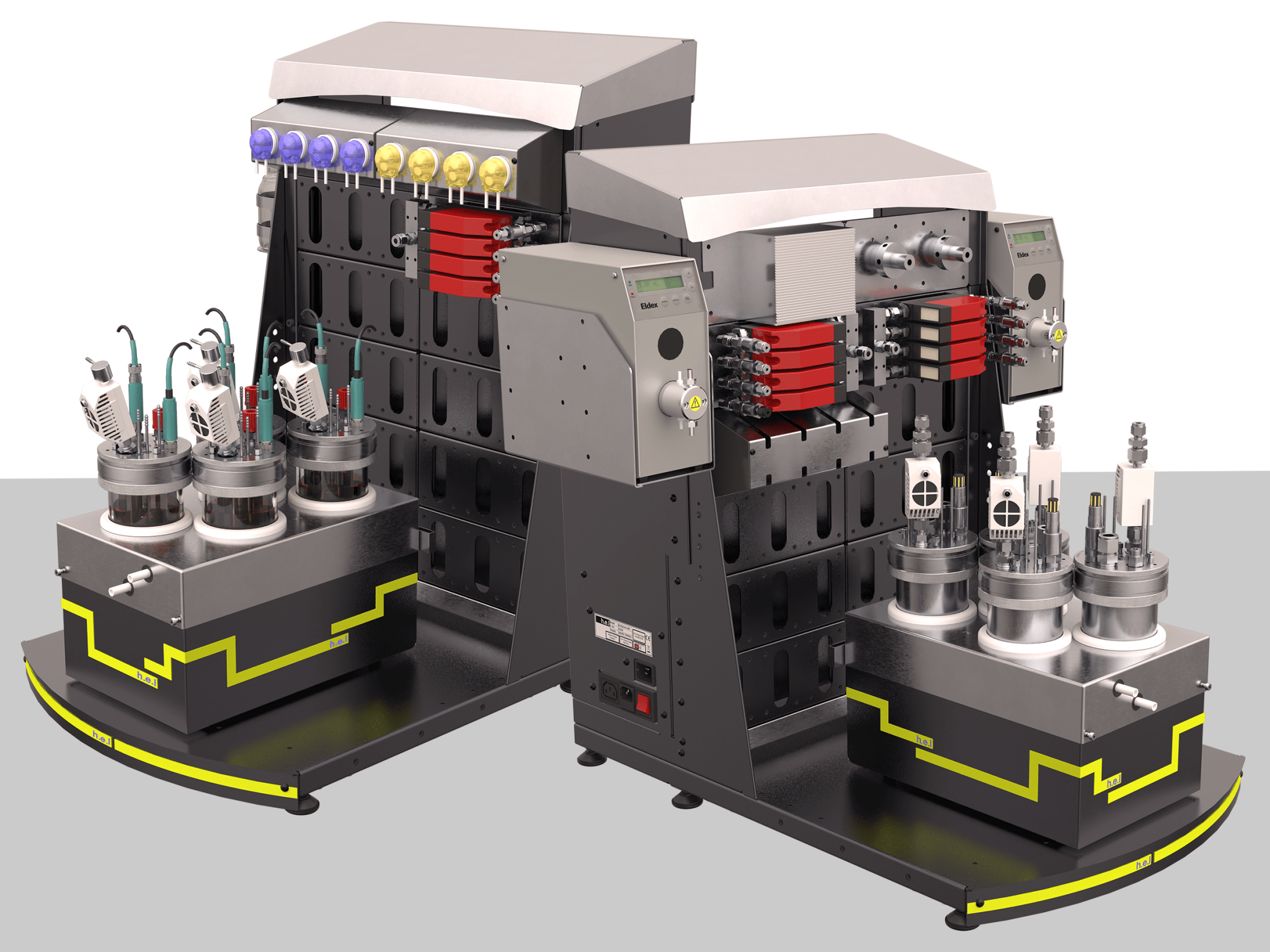
H.E.L’s BioXplorer 400 and 400P
H.E.L Group (London, UK), a global developer and manufacturer of innovative laboratory tools for process optimization, safety, and scale-up, announced the extension of its global sales network with the appointment of two distributors: Paralab and ProAnalytics. The new application specific distributors increase H.E.L’s sales reach and improve access to technical and field application support for biotechnology and chemical synthesis customers in Europe and the US.
H.E.L’s range of BioXplorer bioreactors will be available in the US through ProAnalytics, a company dedicated to providing excellent process analytical technology solutions. Paralab, a distributor of scientific equipment for laboratory and industrial applications, will supply H.E.L’s BioXplorers and PolyBLOCK Compact Automated Parallel Synthesis Platform in Spain and Portugal.
Clinical Trials
VALANX Biotech announces promising results in first in-vivo study of novel protein conjugate therapy
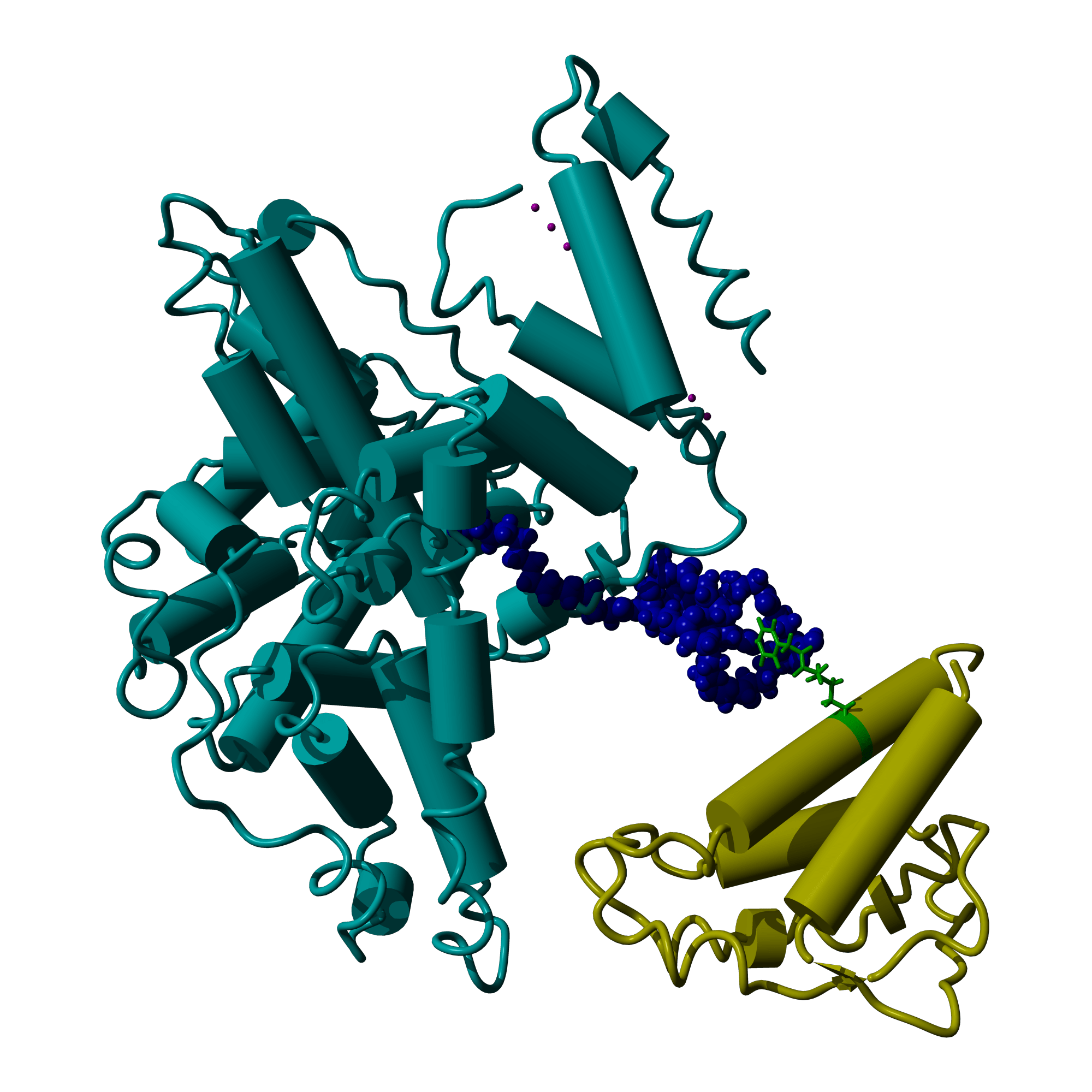
Site specific protein conjugation
VALANX Biotech (Klosterneuburg, Austria), a biotech company developing novel technology for site-specific protein conjugation in drug and diagnostics discovery, announced successful in-vivo results in the Company’s lead program VLX101 for the treatment of a wide range of autoimmune diseases. VALANX’ findings demonstrate the potential of VLX101, a novel Interleukin-2 conjugate and the efficacy of VALANX’ proprietary technology platform and therapeutic approach. VALANX is researching and developing protein expression systems that allow the Company’s own synthetic amino acids to be incorporated into any protein at freely selectable positions in multiple copies.
People
Closed Loop Medicine appoints Paul Johnson as Non-Executive Director
Sphere Fluidics appoints Curtis Nicholson as Director of Sales, EMEA, to support accelerated growth in key markets
Exonate appoints Dr Rafiq Hasan as Non-Executive Director
May update
This month’s Business of Biotechniques highlights industry news, including drug discovery technology partnerships, and products to support cell and gene process development. We also detail companies that have secured investment and financing to progress early disease detection and precision-medicine, and news of appointments within biotechnology and clinical-stage companies.
CRISPR
ERS Genomics and IRBM sign CRISPR/Cas9 license agreement
ERS Genomics (Dublin, Ireland), the CRISPR licensing Company, and IRBM (Rome, Italy), a leader in the field of drug discovery, announced a non-exclusive CRISPR/Cas9 license agreement. The agreement grants IRBM access to ERS’ CRISPR/Cas9 patent portfolio. IRBM is a drug discovery CRO with expertise ranging from target validation and hit finding to preclinical candidate nomination across various therapeutic areas, including oncology, infectious diseases, and neuroscience. The company has contributed to the discovery and development of four marketed therapeutics for HIV, HCV, ovarian cancer, and cutaneous T-cell lymphoma.
Drug discovery & development
Nuclera joins Tech Nation’s Future Fifty Program
Nuclera (Cambridge, UK), the biotechnology company enabling rapid protein expression and purification screening through its eProtein Discovery benchtop protein platform, has been selected to join the Tech Nation Future Fifty 2024 cohort, a unique program designed for the UK’s next generation of unicorn founders, recognizing the most promising late-stage technology ventures. This year’s Future Fifty cohort includes some of the best and brightest ventures across biotechnology, quantum computing, and artificial intelligence. Nuclera, alongside bit.bio, join the program as the first biotechnology companies in the history of Future Fifty.
Metrion Biosciences enhances High Throughput Screening services with access to Enamine compound libraries
Metrion Biosciences (Cambridge, UK) the specialist ion channel and cardiac safety screening contract research organization and drug discovery company, and Enamine (Kyiv, Ukraine), the global leader in supplying small molecules and early drug discovery services, announced that Metrion has enhanced its High Throughput Screening services with the addition of access to Enamine’s compound libraries.
The Enamine compound library collection is the largest in the world and includes both CNS and ion channel focused target libraries. The libraries can be split into discrete screening sets, enabling increased flexibility and efficiencies in screening and target identification. In addition, ‘analog-by-catalog’ from Enamine in-stock and ‘make-on-demand REAL libraries’ present a fast and economical solution for hit expansion and structure-activity relationships studies.
Neurolentech signs technology access partnership with Kaerus Bioscience to advance neurodevelopmental disorder research
Neurolentech (Klosterneuburg, Austria), a pioneering drug discovery startup spun out of IST Austria and focused on epilepsy and related genetic neurodevelopmental disorders (NDDs), announced a technology access partnership with Kaerus Bioscience, a biotechnology company committed to turning scientific advances into treatment realities for patients with rare genetic syndromes of neurodevelopment. The agreement enables Kaerus Bioscience to access Neurolentech’s NDD Drug Discovery platform and leverage its proprietary cell models and assays for functional screens to drive advances in neurodevelopmental disorders research.
Built for NDD drug discovery, Neurolentech’s Platform comprises a vast collection of human-derived neuronal cell lines from patients with monogenic and complex neurodevelopmental disorders. The Platform will enable Kaerus Bioscience to model the complex neural networks underlying clinical features of genetic NDDs at a cellular level, as well as to investigate the therapeutic potential of its small molecule pipeline for numerous genetic syndromes preclinically.
Preci and Biopredic International partner for higher performance of suspended pooled hepatocytes with extended longevity and large-scale availability
Preci (Kyiv, Ukraine), a biotechnology company advancing patient-derived cell models to bridge the gap between preclinical testing and clinical trials, and Biopredic International, a biotechnology company specializing in the design and manufacture of human and animal in vitro assay systems, have collaborated for the production of pooled suspension human hepatocytes. Under a license agreement, Biopredic will leverage Preci’s expertise and production capacity in sourcing primary hepatocytes, and combine with its own IP and know-how in cell pooling. The partnership will provide DMPK researchers access to large batches of high performing suspended pooled hepatocytes with extended longevity from multiple donors.
Semarion introduces SemaCyte Multiplexing Platform to enhance cell assay data quality and speed during drug discovery
 Semarion (Cambridge, UK), a University of Cambridge spin-out company from the Cavendish Laboratory combining materials engineering and cell biology to tackle unmet drug screening needs, introduced its SemaCyte® Multiplexing Platform, an expansion to the existing SemaCyte Microcarrier platform to utilize optical barcoding to accelerate screening processes during in vitro drug discovery. Designed specifically to augment microplate-based high-content imaging approaches, the platform enables in situ multiplexing of adherent cells using the Company’s proprietary microcarriers, SemaCytes, to enhance both the quality and speed of data generation
Semarion (Cambridge, UK), a University of Cambridge spin-out company from the Cavendish Laboratory combining materials engineering and cell biology to tackle unmet drug screening needs, introduced its SemaCyte® Multiplexing Platform, an expansion to the existing SemaCyte Microcarrier platform to utilize optical barcoding to accelerate screening processes during in vitro drug discovery. Designed specifically to augment microplate-based high-content imaging approaches, the platform enables in situ multiplexing of adherent cells using the Company’s proprietary microcarriers, SemaCytes, to enhance both the quality and speed of data generation
Diagnostics
Bio-Rad announces collaboration agreement with Oncocyte to commercialize transplant monitoring with droplet digital PCR
Bio-Rad Laboratories (Hercules, USA), a leader in life science research and clinical diagnostic products, announced a collaboration agreement with Oncocyte Corporation, a precision diagnostics company, to develop and commercialize transplant monitoring products using Bio-Rad’s Droplet Digital™ PCR instruments and reagents. Under the terms of the agreement, Bio-Rad has committed to participate in a private placement of Oncocyte’s equity and has secured exclusive commercial rights in certain markets to commercialize Oncocyte’s assay for transplant monitoring research using Bio-Rad’s QX600™ ddPCR System.
Bio-Rad Laboratories also announced a collaboration with Allegheny Health Network, a western Pennsylvania–based integrated healthcare system. This research collaboration aims to generate clinical evidence across a range of cancer types to support the implementation of Bio-Rad’s Droplet Digital™ PCR technology for tumor-informed molecular residual disease monitoring of patients with solid tumor cancer following curative-intent treatment.
Cell & gene
Broken String Biosciences and the Francis Crick Institute collaborate to advance ALS research
Broken String Biosciences (Cambridge, UK), a genomics company driving development of the next generation of more precise, safe, and effective cell and gene therapies, has entered a research collaboration with the Francis Crick Institute, a world-leading biomedical discovery institute dedicated to understanding the biology underlying health and disease. The project aims to develop novel applications for Broken String’s proprietary DNA break-mapping platform, INDUCE-seq™, beyond its established capabilities in gene-editing. The research will be focused on leveraging the technology to investigate the impact of genomic instability in the development of amyotrophic lateral sclerosis (ALS).
ArgusEye introduces AugaOne to accelerate downstream bioprocess development
ArgusEye (Linköping, Sweden), a provider of innovative sensor solutions for real-time monitoring of biological systems, announced the introduction of the AugaOne™ sensor system. AugaOne is the first product in the Company’s sensor system platform, Auga™, and is tailored to accelerate downstream monoclonal antibody process development by providing specific real-time and automated in-line data with high sensitivity, without requiring sample pretreatment.
Primarily developed to support biopharmaceutical scientists in the detection and quantification of mAbs, AugaOne can handle complex samples such as cell culture and plasma, delivering robust, accurate data independent of matrix effects, cells, and temperatures, with the plan to support process development of other biomolecules in the future. Additionally, its in-line monitoring capabilities greatly reduce the need for time-consuming, manually performed off-line analysis, significantly reducing production lead times.
Asimov achieves 10x improvement in lentiviral production, launches new stable cell line development service
 Asimov (Boston, USA), the synthetic biology company advancing the design and manufacture of therapeutics, introduced the LV Edge Producer System, achieving a 10x improvement in lentiviral production. Available through a fully-stable cell line development service, the system integrates stable and inducible cell lines with software design tools, achieving high lentiviral titres for therapeutic transgenes and enhanced scalability for large-scale lentivirus manufacture. The new streamlined process reduces cost and supply chain risk for cell and gene developers by eliminating the need for complex transient transfection protocols, with the service taking less than six months from sequence to transfer.
Asimov (Boston, USA), the synthetic biology company advancing the design and manufacture of therapeutics, introduced the LV Edge Producer System, achieving a 10x improvement in lentiviral production. Available through a fully-stable cell line development service, the system integrates stable and inducible cell lines with software design tools, achieving high lentiviral titres for therapeutic transgenes and enhanced scalability for large-scale lentivirus manufacture. The new streamlined process reduces cost and supply chain risk for cell and gene developers by eliminating the need for complex transient transfection protocols, with the service taking less than six months from sequence to transfer.
Finance and funding
CN Bio raises $21 million USD in first close of Series B investment round
CN Bio (Cambridge, UK), a leading provider of single- and multi-organ microphysiological systems, has raised a $21 million investment in the first close of its Series B fundraising round. The funding has been secured from several new investors; including $10 million from Bayland Capital, and $5.5 million from founding shareholder, CN Innovations Holdings Ltd. The investment will be used to accelerate the Company’s expanding product portfolio and to scale the business to support commercial expansion across key global markets.
PlaqueTec closes $8 million equity financing
PlaqueTec (Cambridge, UK), a company identifying endotype-specific biomarkers to advance precision medicine for coronary artery disease (CAD), has secured $8 million USD equity financing, led by Lord Moynihan of Chelsea alongside the Future Fund, with support from existing investors. The funding will be used to support PlaqueTec’s ongoing BIOPATTERN trial, designed to improve understanding of CAD pathobiology and how it varies between individuals, and to build BIOCARTA®, its novel biomarker discovery tool.
Owlstone Medical secures $6.5 million to support development of breath-based diagnostics for infectious disease
Owlstone Medical (Cambridge, UK), the global leader in Breath Biopsy® for applications in early disease detection and precision medicine, secured funding from the Bill & Melinda Gates Foundation. The funding is comprised of a $5 million equity investment to advance Owlstone’s Breath Biopsy platform and $1.5 million in grant funding to develop breath-based diagnostics and identify breath biomarkers for tuberculosis (TB) and HIV.
Owlstone, with support from the foundation, is interested in developing new cost-effective detection technologies for volatile organic compounds that could serve as markers of diseases that disproportionately affect the developing world.
People
ERS Genomics appoints John E Milad as Chief Executive Officer
Enhanc3D Genomics appoints Hazel Jones as Chief Executive Officer
Sphere Fluidics appoints Dale Levitzke as Chief Executive Officer and Edward Rayner as Non-Executive Director
Nuclera appoints Joseph Bertelsen as Chief Commercial Officer
Calluna Pharma appoints Margrethe Sørgaard as Senior Vice President of Clinical Operations and Pharmacovigilance
March update
In the latest Business of BioTechniques, we highlight industry news advancing AI-powered drug design, products supporting the safe and effective production of cell and gene therapies, as well as company financing rounds aiming to advance disease treatments. Our round-up also outlines clinical trial news, along with senior management and board appointments at several UK-based companies.
CRISPR
ERS Genomics and StemSight sign CRISPR/Cas9 license agreement
ERS Genomics (Dublin, Ireland), which was formed to provide broad access to the foundational CRISPR/Cas9 intellectual property co-owned by Emmanuelle Charpentier, announced a non-exclusive CRISPR/Cas9 license agreement. The agreement grants StemSight (Tampere, Finland) access to ERS’ CRISPR/Cas9 patent portfolio. StemSight is a preclinical biotechnology company developing off-the-shelf cell therapies for unmet medical needs in corneal blindness. Led by a team of expert scientists in the field of stem cells and tissue engineering for eye applications, StemSight originated as a spin-out from pluripotent stem cell pioneer Heli Skottman’s laboratory at Tampere University (Finland). StemSight is at the forefront of innovative research in regenerative medicine of the cornea.
Drug discovery & development
PlaqueTec and the Babraham Institute collaborate on phenotypic screen of coronary artery blood
PlaqueTec (Cambridge, UK), a company identifying endotype-specific biomarkers to advance precision medicine for coronary artery disease (CAD), based at the Babraham Research Campus, and the Flow Cytometry Facility at the Babraham Institute (Cambridge, UK) have collaborated to develop a cell phenotyping assay to detect cell subpopulations in human blood. Once validated, the bespoke assay will be available as a service in the Flow Cytometry Facility. The collaboration then aims to use the assay to investigate the different cell types present in coronary artery samples collected from patients with CAD consenting to participate in PlaqueTec’s ongoing BIOPATTERN trial.
The collaborative project aims to utilize the assay to perform cell phenotyping analysis on coronary artery samples obtained from patients in the BIOPATTERN trial using PlaqueTec’s unique sampling device, the Liquid Biopsy System™. This exploratory analysis could uncover novel biological insights into the cell types accumulating at coronary disease sites, and the resulting data will be integrated with other multi-omics and imaging data collected in the BIOPATTERN trial to better inform precision approaches to CAD treatment.
Yokogawa introduces CellVoyager High-Content Analysis System CQ3000
Yokogawa Electric Corporation (Tokyo, Japan) introduced CQ3000, a high-content analysis system for capturing high-definition 3D microscopic images of live cell cultures. Expanding the company’s CellVoyager™ family of products, the CQ3000 will be launched commercially later in 2024. The CQ3000 has been designed to capture 3D microscopic images of live cell cultures in high definition at high speed. When used together with Yokogawa’s CellPathfinder image analysis software, the CQ3000 can quantify and analyze intracellular organelles to assess cellular reactions and the effects of drug compounds. It enables highly efficient evaluation of cells in a wide range of applications, from basic research to drug discovery screening.
Software and AI
Closed Loop Medicine demonstrates application of novel drug plus software product for personalized treatment of hypertension
Closed Loop Medicine (London, UK), a leading TechBio company developing combination prescription drug plus software therapy products that enable personalized dose optimization, announced the publication of a peer-reviewed study in the Journal of the American Heart Association. The study describes results of the PERSONAL-CovidBP clinical trial; demonstrating the capabilities of the company’s integrated precision care solution, CLM-HT01, to successfully control blood pressure whilst minimizing side effects and supporting medication adherence in participants with primary hypertension.
Optibrium launches a metabolism prediction software platform tailored to DMPK scientists
Optibrium (Cambridge, UK), a leading developer of software and AI solutions for drug discovery, announced the launch of Semeta™, a metabolism prediction platform tailored specifically for drug metabolism and pharmacokinetics (DMPK) scientists. Fundamental to improving a drug’s chance of clinical success, Semeta allows the accurate prediction of Phase I and II metabolic routes, sites, products and liabilities in early drug discovery, with superior precision to comparable software.
In February, Optibrium announced the release of StarDrop 7.6, the latest version of its platform for small molecule design, optimization and analysis. As part of the release, Optibrium introduces a new extension, Idea Tracker, with which medicinal chemists can now easily trace molecular design and optimization decisions through every step of the complex discovery process, from idea conception to selecting candidates for synthesis.
Cell & gene
Bio-Rad launches Vericheck ddPCR Replication Competent Lentivirus and Replication Competent AAV Kits for cell and gene therapy production
Bio-Rad Laboratories (Hercules, USA), a leader in life science research and clinical diagnostic products, announced the recent launch of the Vericheck ddPCR™ Replication Competent Lentivirus Kit and the Vericheck ddPCR Replication Competent AAV Kit. The kits provide rapid, cost-effective solutions for the absolute quantification of replication competent lentivirus (RCL) and replication competent adeno-associated virus (RCAAV), supporting the safe and effective production of cell and gene therapies.
Asimov launches LV Edge Packaging System to optimize lentivirus production
Asimov (Boston, USA), the synthetic biology company advancing the design and manufacture of therapeutics, launched their LV Edge Packaging System, which improves the cost efficiency and reduces the supply chain risk of lentiviral production. The ready-to-transfer system minimizes GMP plasmid cost, process complexity and supply chain risk by stably integrating viral genes into the host cell. This enables lentiviral production from a single-plasmid transfection, in contrast to the standard four-plasmid process.
In lentiviral manufacturing, GMP plasmids account for a substantial proportion of raw material costs. Procurement of these plasmids also introduces additional supply chain risk and process complexity, which can impact both timelines and product variability. By removing the need to transiently transfect three out of the four GMP plasmids, the LV Edge Packaging System reduces manufacturing cost and supply chain risk without compromising speed to market.
Finance and funding
Calluna Pharma launches and announces €75 million Series A financing to develop novel therapies for inflammatory and fibrotic diseases
Oxitope Pharma (Naarden, The Netherlands) and Arxx Therapeutics (Oslo, Norway), companies with a shared goal of leveraging the innate immune system to discover and develop disease modulating therapies, merged to form Calluna Pharma Inc. (Oslo, Norway). Calluna has raised €75 million in a series A financing and is backed by Oxitope and Arxx’s existing lead investors, Forbion (Naarden, The Netherlands), Sarsia (Bergen, Norway), p53 (Stavanger, Norway) and Investinor (Trondheim, Norway). The new company will combine expertise in the field of innate immunity, based on damage-associated molecular patterns (DAMPs), with a pipeline of selective antibodies that target inflammatory and fibrotic indications, including several first-in-class clinical candidates.
Calluna is developing novel therapies that harness the transformative potential of the body’s immune system. The company’s unique approach involves precision targeting of upstream innate immune amplifiers, enabling disruption of a comprehensive range of disease-associated downstream signaling pathways while maintaining a favorable safety profile.
Enterprise Therapeutics closes £26 million ($33.1 million) series B follow-on financing
Enterprise Therapeutics (Brighton, UK), a biopharmaceutical company dedicated to the discovery and development of novel therapies to improve the lives of patients suffering from respiratory disease, has closed a £26 million (USD$33.1 million) Series B follow-on financing round, led by Panakes Partners (Milan, Italy). Existing investors Versant Ventures (CA, USA), Novartis Venture Fund (MA, USA), Forbion (Naarden, The Netherlands), Epidarex Capital (MD, USA) and IP Group (London, UK) also participated. Alongside the financing, Rob Woodman, Partner at Panakes, joins Enterprise’s Board of Directors. The investment will fund the Phase IIa clinical trial of the company’s lead program, ETD001, to deliver clinical proof-of-concept to treat cystic fibrosis.
MIP Discovery closes £7 million Series A financing to drive commercialization in cell and gene therapy space
MIP Discovery (Bedfordshire, UK), an innovative developer of non-biological affinity reagents designed to accelerate the development and production of cell and gene therapies, closed a £7 million (~USD$9 million) Series A financing round, led by Mercia Ventures (Henley-in-Arden, UK). Existing investor Calculus Capital (London, UK) also participated in the round, along with Angel investors. The investment marks a pivotal change for MIP Discovery as the Company refocuses its mission on improving the downstream processing of cell and gene therapies, to accelerate widespread adoption of these potentially life-changing medicines.
Clinical trials
SolasCure publishes Phase IIa clinical trial report in leading wound care journal
SolasCure (Cambridge, UK), a biotechnology company developing a novel treatment to transform chronic wound care, announced the publication of its CLEANVLU Phase IIa clinical trial report in the International Wound Journal, a leading wound care journal. SolasCure’s first investigational product, Aurase Wound Gel, is a hydrogel releasing tarumase (provisional INN), a recombinant enzyme derived from medical maggots, which aims to promote wound healing through debridement and wound bed preparation. The Phase IIa data, which demonstrates proof-of-concept and safety of Aurase Wound Gel in humans, has now been peer-reviewed and published, providing strong validation as SolasCure progresses into further clinical studies, and marks a significant milestone for the company.
People
Cellular Origins (Cambridge, UK), a TTP Company, appoints Peter Crossley as Chief Operating Officer and establishes advisory board of global industry leaders
Enhanc3D Genomics (Cambridge, UK) appoints Daniel Turner as Chief Scientific Officer
February update
This month’s Business of BioTechniques showcases industry news within the precision medicine, AI-powered drug discovery and synthetic biology sectors. Also highlighted are company collaborations producing new biopharmaceutical technologies, clinical trial programs progressing novel therapies and treatment approaches, as well as news of senior management and board appointments.
CRISPR
Key Charpentier/Doudna CRISPR patent upheld by Japanese Patent Office
ERS Genomics (Dublin, Ireland), which was formed to provide broad access to the foundational CRISPR/Cas9 intellectual property co-owned by Emmanuelle Charpentier, announced that its second Japanese Patent (JP6692856) was upheld for the second time in response to an invalidation challenge. The patent, filed by Charpentier together with The Regents of the University of California (CA, USA) and University of Vienna (Austria), was also upheld previously by the Japanese Patent Office 2021.
ERS Genomics provides licensing of CRISPR/Cas9 technology for companies interested in pursuing its use in their commercial programs. With 89 patents held in over 90 countries, ERS Genomics licenses these patents via its direct license from Emmanuelle Charpentier and now has nearly 150 licenses in place worldwide.
Drug discovery & development
CN Bio PhysioMimix Organ-on-a-Chip data supports Inipharm’s INI-822 for metabolic liver disease treatment: now in clinical testing
CN Bio (Cambridge, UK), a leading Organ-on-a-Chip company that designs and manufactures single-and multi-organ microphysiological systems, announced its PhysioMimix® assay for non-alcoholic steatohepatitis (NASH) was used to provide human-relevant data on compound efficacy for the submission of INI-822 from Inipharm, a biopharmaceutical company focused on discovering and developing therapies for severe liver diseases. The use of in vitro OOC for early evidence of efficacy for INI-822 demonstrates the transformative potential of these models to provide human-relevant data within preclinical programs.
Gyros Protein Technologies and Biotage partner to advance peptide purification efficiency with new automated solution
 Gyros Protein Technologies (Uppsala, Sweden), a leading provider of peptide synthesizers and reagents and a pioneer in automated nanoliter-scale immunoassays, and Biotage, a global life sciences company providing high-quality purification and sample preparation solutions, announced a partnership to offer Biotage® PeptiPEC, based on Gyros’ PurePep® EasyClean (PEC™) catch and release technology and on Biotage® Extrahera™ automated sample preparation system. The development of an automated plate-based peptide purification workflow provides a fast and environmentally sustainable solution for high-throughput peptide purification.
Gyros Protein Technologies (Uppsala, Sweden), a leading provider of peptide synthesizers and reagents and a pioneer in automated nanoliter-scale immunoassays, and Biotage, a global life sciences company providing high-quality purification and sample preparation solutions, announced a partnership to offer Biotage® PeptiPEC, based on Gyros’ PurePep® EasyClean (PEC™) catch and release technology and on Biotage® Extrahera™ automated sample preparation system. The development of an automated plate-based peptide purification workflow provides a fast and environmentally sustainable solution for high-throughput peptide purification.
Lario Therapeutics receives “Company Making a Difference Award” from CDLK5 Forum, recognizing its unique approach to precision medicine for genetic epilepsies
Lario Therapeutics (Edinburgh, UK), a biopharmaceutical company developing first-in-class precision medicines that are targeting disease-modifying treatments for severe neurological disorders, has received the “CDLK5 Forum Award for Excellence – Company Making a Difference 2023 Pre-clinical” from the Loulou Foundation at the annual CDLK5 Forum. The award is in recognition of its development of a validated, precision medicine approach for genetic epilepsies. Lario Tx’s first-in-class, orally active, CNS-penetrant CaV2.3 ion channel inhibitors hold promise as novel anti-seizure therapeutics for multiple epilepsy subtypes, including CDLK5 Deficiency Disorder.
Software and AI
DeepMirror launches early access program for its intuitive molecular drug design software
DeepMirror (Cambridge, UK), a University of Cambridge spin-out company developing intuitive design software for the discovery of novel therapeutic drugs, launched its Early Access Program after a successful closed beta program during which chemists were invited to test the software over several months. The software allows users to tap into AI-driven insights to improve and accelerate molecular design across the drug discovery pipeline through a secure and user-friendly interface which makes AI-powered drug discovery as simple as using a spreadsheet.
AI-enabled drug discovery programs often start with pharmaceutical companies partnering with AI companies to deliver insights for their drug discovery efforts. However, this approach requires extensive crosstalk between the two parties, resulting in long waiting times and large amounts of resources spent on both sides. DeepMirror aims to solve this issue by enabling R&D teams to carry out AI-driven research from day one, with seamless workflow integration and without the need to engage external stakeholders, develop internal teams or software, or relinquish any intellectual property. To join the waitlist for the Early Access Program sign up here.
Optibrium’s quantum mechanics and machine learning methods predict routes of drug metabolism
Optibrium (Cambridge, UK), a leading developer of software and AI solutions for drug discovery, announced the publication of its peer-reviewed study in Xenobiotica, ‘Predicting routes of phase I and II metabolism based on quantum mechanics and machine learning’. In the paper, the team demonstrate a new method that better determines the routes of metabolism and metabolites in early drug discovery.
Based on the combined model outputs, Optibrium showcase a new method to determine the most likely routes of metabolism and metabolites to be observed experimentally. The paper demonstrates that this method delivers high sensitivity in identifying experimentally reported metabolites, as well as higher precision than other methods for predicting in vivo metabolite profiles. It enables researchers to identify compounds with greater metabolic stability and better safety profiles, and underpins Optibrium’s recently launched StarDrop Metabolism module.
Synthetic biology
Evonetix announces agreement for revolutionary gene synthesis platform with Analog Devices
 Evonetix (Cambridge, UK), a company developing semiconductor scale technology to improve access to gene synthesis, has signed a joint development agreement and commercial supply agreement with Analog Devices, Inc. (ADI), a global semiconductor leader. This agreement signals the continuation of the long-standing combined work of both companies, bringing efforts to the next stage in producing a new technology benchmark that will help meet the global demand for better gene synthesis.
Evonetix (Cambridge, UK), a company developing semiconductor scale technology to improve access to gene synthesis, has signed a joint development agreement and commercial supply agreement with Analog Devices, Inc. (ADI), a global semiconductor leader. This agreement signals the continuation of the long-standing combined work of both companies, bringing efforts to the next stage in producing a new technology benchmark that will help meet the global demand for better gene synthesis.
Finance and funding
Metrion Biosciences closes £3.7m new equity financing
Metrion Biosciences (Cambridge, UK), a specialist ion channel CRO and drug discovery company, has secured £3.7m in new equity financing, including £2.5m from lead investor Maven Capital Partners (Maven) and £1m from existing investor, Gresham House Ventures (Gresham House). The funding will be used to further expand Metrion’s laboratories in Cambridge, invest in specialist equipment and enhance the company’s global marketing activities.
Metrion Biosciences also announced a series of board changes. David Milroy has joined the board as Maven’s investor director and Steve Carle has replaced Maya Ward as Gresham’s board representative. At the same time, Marc Rogers, Barry Kenny and Mark Keogh have retired as non-executive directors of the company.
Clinical trials
PlaqueTec recruits first ten patients in BIOPATTERN trial
PlaqueTec (Cambridge, UK), a company identifying endotype-specific biomarkers to advance precision medicine for coronary artery disease (CAD), has received the UK’s Medicines and Healthcare products Regulatory Agency approval to continue its BIOPATTERN trial following recruitment of the first ten patients with CAD. The trial has been designed to improve understanding of the pathobiology of atherosclerotic cardiovascular diseases and how it varies between individuals, to aid the development of novel therapies and treatment approaches.
The BIOPATTERN trial will use PlaqueTec’s proprietary blood sampling device, the Liquid Biopsy System™, to collect samples at multiple sites along a patient’s diseased coronary artery. Thousands of proteins and other blood molecules will be measured in each sample, enabling the assessment of trans-plaque gradients between samples. These data will be analyzed and used to generate a more detailed picture of the disease and enhance clinicians’ understanding of which proteins and biomolecules play key roles in CAD progression towards heart attack.
Exonate announces successful completion of Phase lb/lla trial in diabetic macular edema
Exonate (Cambridgeshire and Nottingham, UK), a biotechnology company developing novel, non-invasive, small-molecule therapeutics for patients with retinal vascular diseases, announced its lead ophthalmology asset, EXN407, has achieved its prespecified endpoints in a phase Ib/IIa study. During the trial, EXN407 met all safety and pharmacokinetic parameters and displayed encouraging signals of biological activity. Exonate has now regained full rights to its complete portfolio of ophthalmology assets from Janssen Pharmaceuticals, Inc. (Janssen), a Johnson & Johnson company. EXN407 is well placed to be the first topical treatment for retinal vascular diseases, including diabetic retinopathy and diabetic macular edema.
Catherine Beech, CEO of Exonate, commented: “The results from the EXN407 trial are very encouraging, with the data validating the hypothesis that modulating VEGF splicing can lead to clinical benefits. We are excited to progress to the phase II trial next year and welcome enquiries by potential partners for the program.”
SolasCure demonstrates proof-of-concept in Phase lla safety trial of Aurase Wound Gel
SolasCure (Cambridge, UK), a biotechnology company developing a hydrogel containing tarumase (provisional INN) – a recombinant enzyme derived from maggots, which aims to accelerate wound debridement and contribute to wound bed preparation & healing – announced the results of its proof-of-concept, first-in-human, Phase IIa safety study. The CLEANVLU trial evaluated the company’s first investigational product, Aurase Wound Gel, for use in chronic venous leg ulcer patients over 12 applications of the product (dosed every 3 days).
People
Sphere Fluidics appoints Dr. Graeme Daniels as Vice President of Sales and Marketing
Broken String Biosciences appoints Gavin Burns as Vice President of Quality and Operations
CN Bio appoints Neil Rumbelow as Director of Product Development
NanoSyrinx appoints Anthony Johnson as Non-Executive Director
Camena Bioscience appoints Dr Nicola Thompson as Chair of the Board
Author details
Zyme Communications provides PR and marketing services for the life science sector to help companies raise their profile and generate interest from commercial leads, investors and partners. With an international network of life science trade media contacts and a strong technical understanding, Zyme is committed to providing real value from objective-led communications. To find out more, visit the website, or follow Zyme Communications on LinkedIn and Twitter.