Updates on Influenza vaccine strain recommended by WHO (2025–2026 northern hemisphere)
Here are the 2025–2026 northern hemisphere Influenza vaccine strain updates.
To maintain vaccine efficacy, WHO regularly updates influenza vaccine strains based on global epidemiological, virological and serological analyses. On 28 February 2025, WHO announced the recommended strains for the 2025–2026 northern hemisphere influenza season1:
-
- Trivalent egg-based vaccines
-
-
- an A/Victoria/4897/2022 (H1N1) pdm09-like virus
- an A/Croatia/10136RV/2023 (H3N2)-like virus
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus
-
-
- Trivalent cell culture- or recombinant protein-based vaccines
-
-
- an A/Wisconsin/67/2022 (H1N1) pdm09-like virus
- an A/District of Columbia/27/2023 (H3N2)-like virus
- a B/Austria/1359417/2021 (B/Victoria lineage)-like virus
-
-
- Quadrivalent egg- or cell culture/recombinant-based vaccines (unchanged from 2024)
-
-
- a B/Phuket/3073/2013 (B/Yamagata lineage)-like virus
-
Comparison of 2024–2025 vs. 2025–2026 vaccine components
Table 1. Key updates of influenza vaccine composition for the 2025–2026 northern hemisphere influenza season.
| Vaccine type | 2024–2025 strains | 2025–2026 updated strains | Key changes |
| Trivalent egg-based | A/Thailand/8/2022 (H3N2) | A/Croatia/10136RV/2023 (H3N2) | H3N2 strain substituted |
| Trivalent cell/recombinant | A/Massachusetts/18/2022 (H3N2) | A/District of Columbia/27/2023 (H3N2) | H3N2 strain substituted |
| Quadrivalent | Tri-valent + B/Phuket/3073/2013* | Tri-valent + B/Phuket/3073/2013* | B/Yamagata lineage retained* |
-
- H3N2 strain substitution
- Egg-based vaccines: 2022 strain (A/Thailand/8/2022) substituted with A/Croatia/10136RV/2023 (H3N2)
- Cell/recombinant vaccines: 2022 strain (A/Massachusetts/18/2022) substituted with A/District of Columbia/27/2023 (H3N2)
- No changes to H1N1 or B/Victoria Lineage
- H1N1 strains (egg: A/Victoria/4897/2022; cell/recombinant: A/Wisconsin/67/2022) and B/Victoria lineage (B/Austria/1359417/2021) remain unchanged
- Summary
- Core change: updated H3N2 strains reflect their high mutation rate
- Continuous development: consecutive adjustments to H3N2 highlight the need for rapid vaccine adaptation to viral evolution
- H3N2 strain substitution
Recombinant antigens: critical materials for vaccine research
Table 2. Key target antigens (HA, NA, NP) play vital roles in influenza vaccine, drug and diagnostic development.
| Protein | Function | Applications |
| HA (Hemagglutinin) | Binds to host cell receptors, mediates viral entry | Vaccine development, neutralizing antibody research, diagnostic reagents |
| NA (Neuraminidase) | Cleaves sialic acid receptors to release viral particles | Antiviral drug development, vaccine optimization, diagnostic kits |
| NP (Nucleoprotein) | Binds viral RNA, supports replication and genome stability | Broad-spectrum antiviral research, diagnostic product development |
Applications of recombinant antigens:
- Vaccine development: used in potency testing, ELISA-based antibody level analysis and quality control
- Research acceleration: replace traditional viral culture methods, shortening R&D timelines
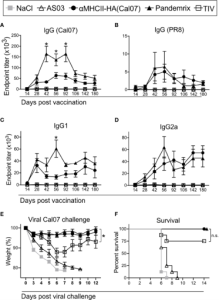
Figure 1. Recombinant HA protein used to measure serum antibody levels in vaccinated mice 2.
Sino Biological unveils comprehensive Influenza research reagents for 2025–2026 vaccine strains
Sino Biological, a leading provider of biological reagents and services, has announced the release of its latest portfolio of influenza research reagents. Designed to support the development of vaccines for the 2025–2026 northern hemisphere influenza season, the new offerings include high-quality recombinant HA, NA and NP proteins. These reagents are now available for immediate order, providing researchers with the tools they need to accelerate their studies and contribute to global influenza prevention efforts. For more details, please visit our website: www.sinobiological.com.

Scan me to order