CRISPR to restore cardiac function
Researchers are now taking CRISPR to the next level, bringing us closer to treating fatal muscular degeneration.
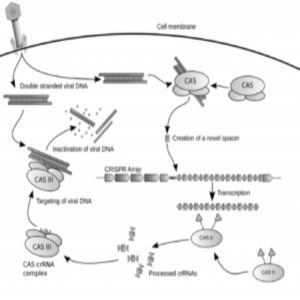
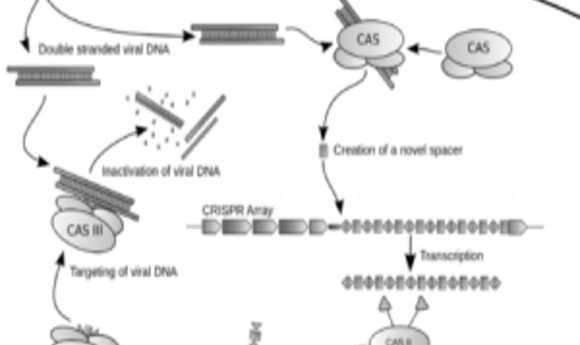
Diagram of the CRISPR prokaryotic antiviral defense mechanism
Credit: Wikimedia
Since 2008, Ohio State University researcher Renzhi Han’s goal has been to perform gene surgery to correct Duchenne muscular dystrophy (DMD), a progressive disease that results in cardiac defects in approximately 95% of patients. These cardiac defects are the leading cause of death for DMD patients. “I was dreaming of one day when muscular dystrophy patients can have treatment–a one-time treatment–which can last for life and allow them to live a quality life,” said Han.
Han hoped to correct the disruptive gene mutations in the dystrophin gene, but the tools simply weren’t available at that time. Then when researchers introduced the CRISPR/Cas9 system in 2013 for editing genes in cultured cells, his dream drew closer to becoming reality. “We realized that CRISPR/Cas9 might be a real deal to fix genetic defects in patients,” said Han. Now he and his team report in Circulation Research that CRISPR-mediated genome editing corrects dystrophin expression and restores cardiac function in dystrophic mice.
Han and his team designed their CRISPR components and packaged them into adeno-associated viral vectors. When systemically delivered to mouse neonates, the CRISPR components efficiently excised the targeted mutations and restored dystrophin protein expression in the mice.
“Our study shows that the gene editing is effective in restoring dystrophin expression in about 40% of cardiomyocytes, and it is expected that this should be effective in alleviating cardiomyopathy and preventing heart failure,” said Han. The team also observed restored cardiac muscle fiber architecture and contractility of cardiac papillary muscles in the ventricles in dystrophin-deficient mice. The mice also had less cardiac tissue thickening and scarring compared with untreated controls.
“If proven to be effective in the clinic, this treatment would be revolutionary as it corrects the genetic defects in the patients’ coding DNA, which has never been possible before,” said Han. Han plans to continue his preclinical work with the hope of gathering enough data to move into clinical testing in the coming years.





