How do Hox genes control the way we look?
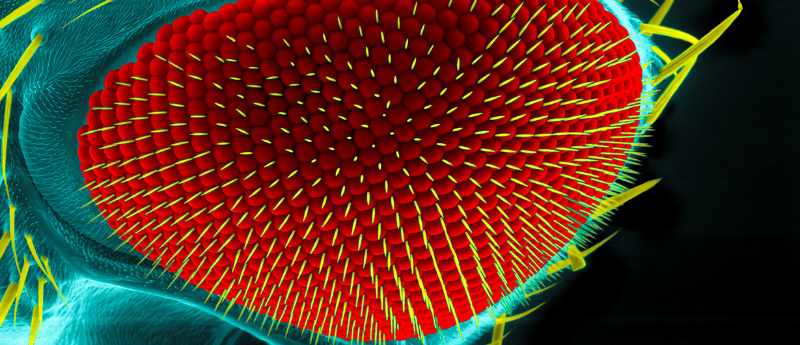
New findings utilizing CRISPR-Cas9 gene editing in drosophila demonstrate the ‘scaffold’ role of Hox genes in the development of anatomical appearance.
Following on from the ‘McGinnis experiment’ 30-years ago, Ankush Auradkar, William McGinnis’ mentee, has led a study on Hox genes, alongside senior author Ethan Bier (both University of California San Diego; UCSD, CA, USA). The researchers used CRISPR-Cas9 gene-editing technology to investigate the role of Hox genes in the development of anatomical facial features of Drosophila flies. The findings have implications for evolutionary biology in Drosophila, but could also help explain developmental issues associated with human genetic processes.
The ‘McGinnis experiment’ took place back in 1990, uncovering how high-level control Hox genes can alter physical anatomical development. The initial research laid down the framework of understanding the role of Hox genes in determining uniform appearances, applicable across all species.
Several fundamental questions remain unanswered since the unearthing of these Hox genes that puzzle evolutionary and developmental biologists alike. First, do the Hox genes provide ‘positional codes,’ acting like a scaffold to regulate downstream expression patterns, or do they themselves instruct morphological changes? Second, in the context of evolution, what is the importance of coding versus regulatory sequence changes?
 Indel detection by amplicon analysis: an alternative sequencing method
Indel detection by amplicon analysis: an alternative sequencing method
Eric Paul Bennett, from The University of Copenhagen, discusses the development of the Indel Detection by Amplicon Analysis (IDAA) technique as an alternative to next-generation sequencing.
In this study, the authors hoped to answer these questions about the function of Hox genes by following in the footsteps of the original McGinnis experiment, which tested whether proteins encoded by the human or mouse Hox gene could function in flies.
After whole-genome extraction, the team sequenced the genome of the common laboratory fruit fly, Drosophila melanogaster (D. mel) and its Hawaiian cousin, Drosophila mimica (D. mim) using a combination of short (Illumina) and long (Sanger) reads. Next, using CRISPR gene editing, the researchers deleted the Hox gene ‘proboscipedia’ (pb) locus from the D. mel genome and replaced it with a copy of the pb gene from D. mim.
The pb gene controls development of mouthpart structures that are easily distinguishable between the two species; D. mel’s mouthpart looks smooth, whereas D. mim’s mouthpart appears more angular.
As hypothesized by the team, the results of the study indicated that D. mel’s smooth mouthparts prevailed over the angular mouthparts encoded by the D. mim pb sequence introduced to the genome. These results demonstrate that Hox genes act as a scaffold for downstream genes that best benefit the organism, rather than directly instructing morphological changes.
Whilst D. mel.’s features generally triumphed, a change in the control region caused a different pattern of pb gene activation to be observed. The maxillary palps (sensory organs) on the face of D. mel usually stick out, but were found to be parallel to the mouthparts as is the case in the D. mim fruit fly. This indicated that pb sequences can evolve in closely related species to alter morphology.
“These insights may lead to a better of understanding of processes tied to congenital birth defects in humans,” explains Bier. “With the advent of powerful new CRISPR-based genome editing systems for human therapy on the horizon, new strategies might be formulated to mitigate some of the effects of these often-debilitating conditions.”