Knockout animals’ secret weapon

Many medical advances of recent years have been possible due to knockout mice. Advances in gene editing techniques have been headline-grabbing but another piece of the method, electroporation, has been just as crucial in these developments.

Knockout, or transgenic, mice are genetically modified lab animals that have had certain genes inactivated, removed or artificially introduced. They have become a fundamental part of research into the function of certain genes, from cancer research, [1] to the study of addiction [2]. If a scientist knows what gene has been knocked out, they can then observe the behavior, health and biomarkers of the mouse to provide clues to the gene’s function to help understand the mechanisms of disease and behavior.
In 1989 Mario R. Capecchi, Martin Evans and Oliver Smithies created the first knockout mouse, which led to them winning the Nobel Prize in Physiology or Medicine in 2007 for their work. Genome editing has received a lot of attention over recent years, with CRISPR/Cas9 regularly making the headlines. However, a slightly overlooked but crucial component of their method was the ability to introduce molecules such as DNA into the cell by breaking through the cells membrane using electroporation.
Electroporation is a method by which electrical fields are applied to cell membranes to increase their permeability. This allows molecules such as DNA to enter cells, via small pores in the membrane created by electroporation. It forms a crucial part of the process of creating knockout mice.
These methods facilitated the creation of knockout mice and all the impactful research that followed; however, there are limitations associated with knockout mice creation. Along with practical issues, such as the time taken for the mice to develop and be phenotyped, there are also the epigenetic effects and changes that can be caused by the rest of the mice genome [3]. However, due to the relative ease in which they can be generated and their ubiquity across research, knockout mice have become the most successful animal model of recent years for genetic study.
Knocking out mice
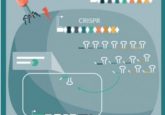
Capecchi and colleagues outlined the broad steps they used to generate these transgenic mice in 2007 [4]. The first two steps in the process; designing; or gene editing, inserting or electroporation, have seen rapid development over recent years.
Designing the target vector involves isolating the gene to be knocked out from a gene library and engineering a new sequence that is sufficiently changed to be inactive but sufficiently similar as to be incorporated into the target mouse sequence. Additionally, marker genes are added that produce observable change that is useful for selection further down the line – in the form of a fluorescent gene, for example. In recent years CRISPR/Cas9, ZFN and TALEN have become the most successful methods for this gene editing [5] in transgenic organism development.
The sequence is then inserted into embryonic stem cells using electroporation. Basic principles of the method have remained; highly charged molecules, such as DNA, are able to permeate the lipid bilayer of the cell due to the creation of small water-filled holes in the membrane. Voltage is applied according to the required threshold of the target cells. This generates a dynamic process whereby the cell membrane flickers between states. This allows scientists to control the permeability of the bilayer and determine whether the membrane will be able to heal after the method, or rupture.
Recently, a team of scientists from Kyoto University and Hiroshima University (both Japan) described a new method – Technique for Animal Knockout system by Electroporation (TAKE) – which generates knockout rodents by directly introducing modified endonucleases, and not embryos, via electroporation [6]. For the electroporation part of TAKE, the scientists used the NEPA 21 electroporator, which uses a three-step pulse system. The first pulse creates holes in the membrane, the second drives the target sequence into the embryo and the third changes the polarity to ensure the whole mRNA sequence is transferred. The scientists believe their method simplifies the electroporation step and produces effective results compared with more traditional methods.
Advances in gene editing, such as CRISPR/Cas9, have required a step up in electroporation methods. Scientists at the University of California, Berkeley, (USA) have developed a new system, called CRISPR-EZ, which has been shown to introduce CRISPR-modified genes into mouse embryos with 100% efficiency [7]. The study, published in the Journal of Biological Chemistry, demonstrates how new gene editing techniques can be combined with electroporation methods to generate transgenic models more efficiently.
Traditionally, with some transgenic CRISPR studies, one step is the microinjection of CRISPR/Cas9 into target embryos. This method is supposed to shorten the transgenic creation process by modifying DNA inside the target embryo itself instead of editing before as described above. However, microinjection can lead to low viability of embryos and is expensive and time consuming. In order to overcome this, the team used electroporation to get CRISPR inside the embryos, which resulted in a far greater number of transgenic mice largely due to the increased viability of the electroporated embryos compared with the microinjected ones.
This method is not only more efficient but also cost-effective. Co-author Russel Vance is excited by the potential of lowering costs and increasing efficiency:
“In the not too distant past, it would cost at least US$25,000 and take at least 6 months to make a knockout mouse. With CRISPR, and improvements such as CRISPR-EZ, the costs and time have both dropped at least 10-fold. These technical innovations make the mouse an even more powerful tool for modeling human diseases.”
It’s not just gene editing, but gene insertion, that has been responsible for a large part of recent developments in knockout animal creation. Crucially, it’s the combination of these rapidly developing fields that have accelerated research around into the world to answer the biggest challenges facing human health today.