More compact CRISPR enzyme engineered

Researchers have modified the AsCas12f enzyme to enhance its gene-editing activities while maintaining its small size.
A collaboration led by researchers at the University of Tokyo (Japan) has engineered a compact CRISPR enzyme using deep mutational scanning, a method for the multiplex measurement of thousands of protein variants, and structural analysis methods. The resulting enzyme, enAsCas12f, is a third of the size of Cas9 and just as effective. This could lead to new and more effective gene therapies.
There is a size limit to what can be packed into an adeno-associated virus (AAVs), and Cas9 is at the very limit of this, so there is a demand to find alternatives that are as efficient as Cas9 but more compact. The researchers of this study looked to the AsCas12f enzyme from the bacteria Axidibacillus sulfuroxidans, which is one of the most compact Cas enzymes known. Whilst compact, this enzyme has previously shown little genome activity in human cells.
To address this limitation, the researchers used deep mutational scanning and “assembled a library of potential new candidates by substituting each amino acid residue of AsCas12f with all 20 types of amino acids on which all life is based,” explained senior author Osamu Nureki. “From this, we identified over 200 mutations that enhanced genome-editing activity. Based on insights gained from structural analysis of AsCas12f, we selected and combined these enhanced-activity amino acid mutations to create a modified AsCas12f.” The resulting enzyme (enAsCas12f) has more than 10 times the genome-editing activity of the unmodified AsCas12f enzyme and is comparable to Cas9. To understand the molecular mechanisms of the enzyme, they used cryo-EM.
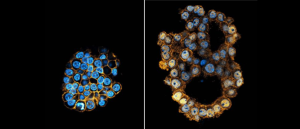
Base editing for more efficient and representative organoids
Using base editing, researchers have been able to establish tumor organoids with multiple mutations more efficiently.
The modified enzyme has been tested in mouse models and shows promise for use as a gene therapy. Its small size means it can be packed into the carrier virus and delivered with greater efficiency.
The team acknowledge that this engineered AsCas12f may not be the most optimal combination of identified mutations. They plan to use computational modeling or machine learning to predict which combinations might offer even greater improvements.
The development of this compact enzyme could lead to more compact genome-editing tools and new gene therapies. “Using the engineered AsCas12f we developed, our next challenge is to actually administer gene therapy to aid people suffering from genetic disorders,” concluded Nureki.