Reining in CRISPR-Cas9 with [C]gRNA
‘Safeguard’ guide RNA regulates CRISPR-Cas9 activity and reduces unwanted mutations.
Whilst genome editing with CRISPR-Cas9 is effective, it runs the risk of off-target effects and excessive mutations, which can lead to toxicity. To reduce the likelihood of these occurring, researchers at Kyushu University (Fukuoka, Japan) and Nagoya University School of Medicine (Japan) have developed a new genome editing platform with a ‘safeguard single-guide RNA’, which could lead to more effective and safer treatments for genetic diseases.
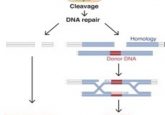
In the CRISPR-Cas9 gene editing system, the Cas9 nuclease is introduced into cells and directed to the location of interest with synthetic guide RNA (gRNA), where Cas9 cleaves the two DNA strands. Off-target effects are often due to the Cas9 enzyme cleaving genomic sites with a similar sequence to the target and can result in mutations including, but not limited to, chromosomal rearrangements and genomic deletions. The research group hypothesized that current CRISPR-Cas9 protocols cause overactivity of the enzyme, resulting in excessive DNA cleavage and unwanted mutations.
To find out if this is the case, the researchers developed an allele-specific indel monitoring system (AIMS) in mouse cells to evaluate the activity of Cas9 in each individual chromosome. This system visualizes the dynamics of genome editing. They concluded that CRISPR-Cas9 does display very high editing activity, resulting in some of the unwanted side effects associated with gene editing.
Next, the researchers wanted to find a way of suppressing this activity by modifying the gRNA. They found that adding an extra cytosine to the 5’ end of the gRNA acted as a ‘safeguard’, reducing overactivity and allowing greater control over the DNA cleavage. They called the modified gRNA ‘safeguard gRNA’, or [C]gRNA.
“For techniques that use Cas9 to activate or repress genes of interest, such as CRISPR activation and CRISPR interference, excessive induction or suppression of gene expression may not be useful and even harmful to cells,” explained Hiroshi Suzuki (Nagoya University School of Medicine), a corresponding author on the study. “Controlling expression levels by [C]gRNA is an important technology that can be used for various applications, including the implementation of precise gene therapy.”
With this new technique, the off-target effects and cytotoxicity were reduced, efficiency of single-allele selective editing was increased and the efficiency of homology-directed repair, the most common mechanism for DNA double-strand repair, was improved.
 Bacteria provide inspiration for novel therapeutic delivery approaches
Bacteria provide inspiration for novel therapeutic delivery approaches
Two different research groups have harnessed natural bacterial systems to deliver therapeutic payloads.
The researchers tested their activity-regulating [C]gRNA by investigating a rare disease called fibrodysplasia ossificans progressive, a disorder where muscle tissue and connective tissue are gradually replaced by bone. This condition is caused by a substitution of a single guanine residue with adenine, which requires the most difficult edit possible to correct: a mono-allelic single-nucleotide substitution. The team demonstrated the ability of the techniques to recreate the same genetic mutation that causes the disease in humans in a mouse model.
As an additional proof of principle, using fibrodysplasia ossificans progressive patient-derived human induced pluripotent stem cells, the researchers successfully repaired the single nucleotide mutation, specifically in the disease-associated allele. With their new approach, the typically observed off-target effects were significantly reduced.
Their results indicate the potential and safety of using their method in gene therapy. Additionally, they showed that [C]gRNA can be used in combination with other CRISPR tools, including Cas12a, which carries out a different cleavage mechanism from Cas9.
In this study, the research group also created the first mathematical model of the correlation between genome-editing patterns and Cas9 activity. This can be used to simulate the effects of genome editing in an entire group of cells, allowing researchers to predict the desired Cas9 activity to maximize efficiency.
The research group is hoping to launch a start-up with its new platform. “In particular, we believe that this technology can make a significant contribution to the medical field,” commented the study’s first author, Masaki Kawamata (Kyushu University). “We are currently evaluating its therapeutic efficacy and safety for selected target diseases in cell and animal experiments and using it to help develop therapeutic drugs and gene therapy methods, especially for rare diseases for which no treatment methods have yet been established.”