US scientists modify human embryos with CRISPR/Cas9
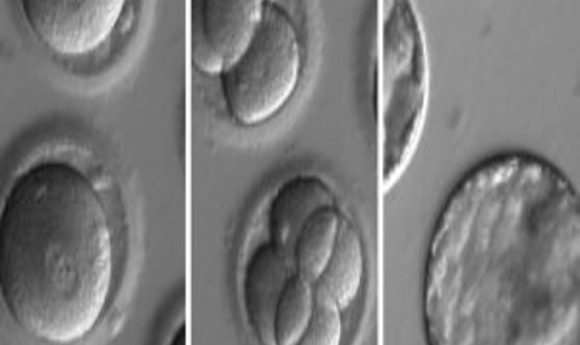
Researchers repaired a harmful mutation by genetically editing human embryos, pushing the world closer to germline clinical trials.
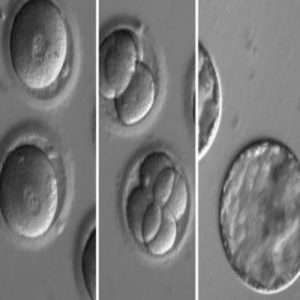
Embryos developing after co-injection of CRISPR components to correct a gene known to cause hypertrophic cardiomyopathy.
Credit: OHSU
In a much anticipated Nature study, embryologist Shoukhrat Mitalipov and colleagues report using CRISPR/Cas9 technology in human embryos to repair a mutation linked to heart disease.
“This is the first large embryonic gene-correction study allowing reasonable conclusions,” said co-author Paula Amato at Oregon Health & Science University. “If proven safe, this method could prevent transmission of genetic disease to future generations.”
Over a week before the study’s publication, leaked contributor names and anonymous tips fueled growing anticipation of these “germline-editing” findings. But without hard data, most early media coverage was limited to speculation.
“We don’t like to use the word ‘editing’; what we actually did was un-modify the original mutant gene using CRISPR/Cas9 and the wild-type maternal gene,” explained co-author Jin-Soo Kim of the Institute for Basic Science in South Korea.
Approximately 40% of familial hypertrophic cardiomyopathy (HCM) cases result from an autosomal dominant mutation in the MYBPC3 gene on chromosome 11. In this study, the researchers used CRISPR/Cas9 to reintroduce four missing base pairs into the mutated MYBPC3 gene.
The Oregon-based team prepared induced pluripotent stem cells from a skin biopsy from a male donor with HCM and targeted the mutated MYBPC3 gene with CRISPR/Cas9. Once the gene was cut by the Cas9 enzyme, the donor’s DNA-repair mechanisms fixed the mutation in the next round of cell division using either a synthetic DNA sequence or the healthy copy of the MYBPC3gene as a template.
Mitalipov’s team then selected the best-performing gene-editing components from this experiment and injected them into healthy donor eggs that had just been fertilized with the male donor’s sperm using in vitro fertilization (IVF) protocols. Then they analyzed cells from early embryos at single-cell resolution to assess repair efficiency.
CRISPR/Cas9 cut DNA at the correct position, and 42 of the 58 embryos (72.4%) did not carry the hypertrophic cardiomyopathy mutation. In the remaining 27.6% of embryos, the cellular cut-repairing mechanism introduced unwanted insertions or deletions near the cut.
“Even though we saw a leap in efficiency, we’re not at 100% yet,” explained Mitalipov. “I’m sure we can improve CRISPR repair to achieve 90%–100% efficiency. Then I think we’d be ready for clinical trials. At this point, it’s not clear how trials would proceed because US regulatory agencies would have to define Phase 1 and Phase 2 requirements.”
Earlier this year, the National Academy of Sciences cautiously opened the door to clinical trials using heritable genome editing, but “only within a robust and effective regulatory framework” and in “the absence of reasonable alternatives,” among other criteria (2).
Previously, two CRISPR/Cas9 applications in human embryos were done in China on a smaller scale. In this study, Mitalipov avoided mosaicism, a problem reported for the earlier studies, by injecting CRISPR/Cas9 into the egg immediately after fertilization. The researchers anticipate that using CRISPR/Cas9 with IVF will be helpful for other monogenetic diseases where a healthy gene copy is available.