Sample, shower, shave: the at-home urine test that could revolutionize prostate cancer screening
A recent study has demonstrated the benefits of at-home testing for prostate cancer tests due to enriched biomarker concentration in urine samples, enabling more effective screening for the disease.
In a study recently published in BioTechniques, researchers from the University of East Anglia (UEA) and the Norfolk and Norwich University Hospital (both Norwich, England) have developed a urine test, capable of utilizing samples provided at home. This has been shown to improve prostate cancer tests when compared to the current standard methods of detection.
The prostate releases secretions continuously and so any biomarkers present in those secretions will build up overnight in an individual’s urine as they experience the longest period between excretions in a 24-hour period. The first urination of the day, therefore, will contain a significantly higher and more consistent level of biomarkers.
Prostate cancer frequently requires monitoring as, according to lead researcher Jeremy Clark (UEA), “it usually develops slowly and the majority of cancers will not require treatment in a man’s lifetime. However, doctors struggle to predict which tumors will become aggressive, making it hard to decide on treatment for many men.”
The prostate cancer test
Considering prostate cancer’s prevalence in society – it is the most common cancer in men in the UK – and with the knowledge of enhanced biomarker prevalence early in the day, Clark set out to develop a test that could effectively process samples collected at home, early in the morning, without the need for patients to travel to provide the sample.
The resultant prostate urine risk (PUR) test also circumvents the need for more invasive, time consuming and uncomfortable diagnostic methods for prostate cancer diagnosis, such as blood tests, biopsy, MRI and digital rectal examination (DRE).
The PUR test measures gene expression in the urine samples, which can be posted to labs for analysis, and can provide key indicators of the malignancy of a tumor.
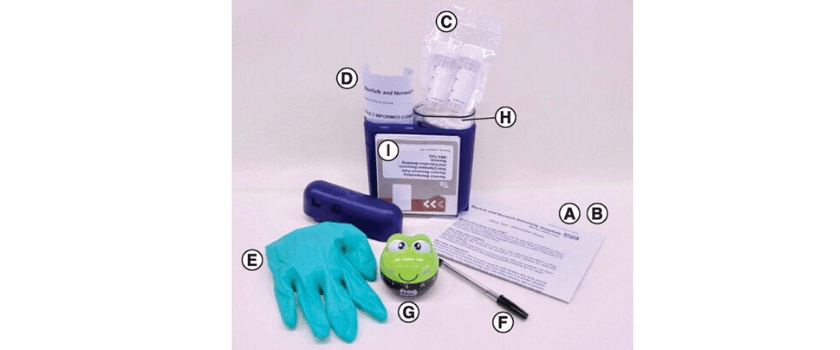
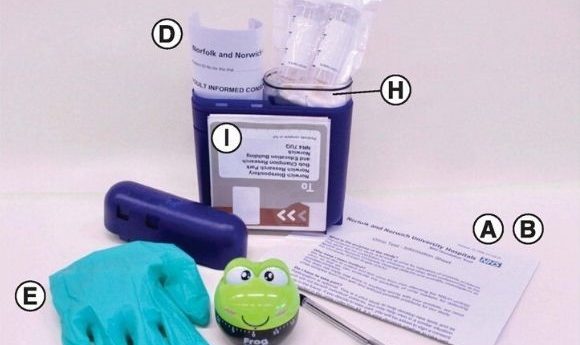
(A) Invitation to participate. (B) Study information sheet. (C) Two urine collection tubes (30 ml) containing dried Norgen preservative. (D) Two consent forms (one for the patient to keep and the other to return with the samples). (E) Disposable non-allergenic glove. (F) Pen (to write the time and date on the tubes). (G) 1-h frog timer. (H) Sealable plastic bag with wadding. (I) Preaddressed postage-paid SafeBox for returning the samples.
- High-dose, high reward? New potential treatment for prostate cancer
- Could a blood test for breast cancer be on the horizon?
- Balkees Abderrahman on exhaled breath biopsy for cancer diagnosis
The study
An ‘At Home Collection Kit’ was distributed to 14 study participants who were instructed in their use. The samples, taken early in the morning, were then compared to samples obtained from the same participants by DRE.
The results demonstrated that the urine samples displayed a clearer picture of the biomarkers present. This was coupled with feedback from the participants, which indicated that the urine sample was, perhaps unsurprisingly, “preferable”.
Commenting on the potential impact of the study, Clark declared that the test could “revolutionize how those on ‘active surveillance’ are monitored for disease progression, with men only having to visit the clinic for a positive urine result. This is in contrast to the current situation where men are recalled to the clinic every 6 to 12 months for painful and expensive biopsies.”
Impact and reception
Consultant urology surgeon Robert Mills, of the Norfolk and Norwich University Hospital, expressed his enthusiasm for the potential of the test to affect the treatment of the 800 plus prostate cancer referrals that the hospital receives each year. “This is a very exciting development as this test gives us the possibility of differentiating those who do from those who do not have prostate cancer so avoiding putting a lot of men through unnecessary investigations.”
Mills also revelled in the ability of the test to make surveillance of the disease more effective, convenient and comfortable for patients that have been diagnosed with the cancer but do not yet require treatment and are therefore on active surveillance programs.
The research team is now hopeful to see if their test can be adapted to diagnose kidney and bladder cancer. Ultimately this research could lead to a significantly improved screening regime for a whole host of cancers, which can be used to screen more patients and easily fit into their morning regimes of sample, shower, shave.





