Cutting-edge technology could revolutionize retinal disease drug development
Refer a colleague
Researchers have engineered a highly developed model of the human retina, known as retina-on-a-chip, which replicates the natural environment and may be useful in helping scientists to study retinal diseases.
Currently, there is a huge demand for the development of novel therapeutic strategies to treat retinal diseases, such as Stargardt disease. However, current models fail to produce results that accurately describe the effects in humans.
Researchers from both the Eberhard Karls University Tübingen (Germany) and the Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB (Stuttgart, Germany) have developed new technology that could revolutionize the development of treatment for these retinal diseases.
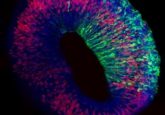
The novel microphysical model, known as retina-on-a-chip (RoC), combines organoid and organ-on-a-chip technology to generate a complex multi-layer tissue model of the human retina that mimics the same environment cells would experience in the human body.
Until now, ophthalmologic drug development has relied heavily on animal models that do not provide results that are translatable to human disease. The development of self-forming retinal organoids derived from human stem cells provided a lot of potential in the area. However, they lack vascularization and the ability to replicate interactions between photoreceptors and retinal pigment epithelium.
The study, published recently in eLIFE, described how – using solely engineering techniques – the researchers overcame these issues by integrating more than seven different essential retinal cell types derived from human pluripotent stem cells into the tissue.
“This combination of approaches enabled us to successfully create a complex multi-layer structure that includes all cell types and layers present in retinal organoids, connected to a retinal pigment epithelium layer,” explained co-lead author Kevin Achberger (Eberhard Karls University of Tübingen). “It is the first demonstration of a 3D retinal model that recreates many of the structural characteristics of the human retina and behaves in a similar way.”
The fact that the RoC enables the interactions between mature photoreceptor segments with the retinal pigment epithelium in vitro allows for the establishment of in vivo-like physiological processes such as outer segment phagocytosis and calcium dynamics.
As well as being damaged by specific retinal diseases, the retina is vulnerable to side effects from novel therapeutic agents to treat other diseases, such as cancer. The RoC would also function to allow drug developers to more accurately test the side effects of those drugs.
In order to test this theory, the team treated the RoC with two drugs known to be toxic to the retina: the anti-malaria drug chloroquine and the antibiotic gentamicin. They found that the drugs produced the same toxic side effects in the retinal cells in the model as they’re known to produce in humans, suggesting that the RoC could be effective in testing this.
“One advantage of this tiny model is that it could be used as part of an automated system to test hundreds of drugs for harmful effects on the retina very quickly,” commented Achberger. “Also, it may enable scientists to take stem cells from a specific patient and study both the disease and potential treatments in that individual’s own cells.”
Please enter your username and password below, if you are not yet a member of BioTechniques remember you can register for free.