‘Death Star’ structure could save lives via improved drug delivery
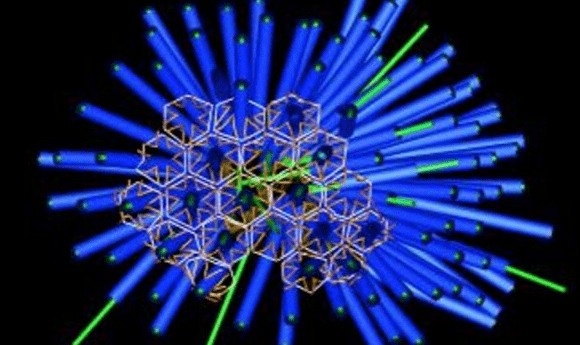
The mechanism of a Death Star-like protein injection system in bacteria has been exposed, highlighting its potential applications in drug delivery.
A team from San Diego State University (SDSU; CA, USA) has further elucidated the mechanism of a ‘death star’-like injection system in bacteria that could prove useful for targeted drug delivery.
The injection system was identified in a bacterium that has a beneficial relationship with tubeworms found on the bottom of boats. However, this ‘good’ relationship has a dark side, resulting in biofouling. Thick, crusty layers of the invertebrates form on the bottoms of boats resulting in increased weight, and subsequently higher fuel consumption. As such, determining the mechanisms of the relationship is of interest to the marine industry, as well as holding interest for drug delivery approaches.
Research had already shown that the tubeworms are attracted to areas with healthy populations of bacteria such as Pseudoalteromonas luteoviolacea as they are conducive to reproduction. The SDSU team determined that the bacteria are essential to the process, injecting content into tubeworm larvae through syringe-like Metamorphosis Associated Contractile structures (MACs), resulting in their transformation into juvenile worms. The MACs appear on approximately one in 50 bacteria when they undergo lysis.
- Parasitic fish helps cancer drugs swim through the blood–brain barrier
- Tumor-targeting protein could be future of personalized cancer therapy
- A postman particle: delivering therapeutics for osteoarthritis
The team went on to use cryo-electron tomography to examine the structures, leading to the discovery of arrays of death star-shaped injection systems that contain Mif1, the protein responsible for metamorphosis. The MAC structure is similar to that which can be found on bacteriophages, and it is possible the structure has been ‘stolen’ from them.
“Phage typically attack bacteria with these structures, but instead of using it to infect other bacteria, the Pseudoalteromonas now uses it to interact with other animals, such as tubeworms, insects, and mouse cells,” explained Nicholas Shikuma (SDSU). “MACs are created when the bacteria undergo cell lysis – when the cells blow themselves up – and the bacteria that do this die afterwards, so it’s almost like altruism because it benefits the rest of the bacterial population.”
MACs have been shown to be capable of targeting many ex vivo eukaryotic cell lines, and thus the microscopic structures theoretically could be modified for drug delivery approaches, for therapeutics or vaccines. Now, the team is examining data from the Human Microbiome Project to determine if humans have a similar structure in the gut.
Time will tell as to whether MACs will prove fruitful for effective drug delivery, a field where accuracy is so important, we must “Do. Or do not. There is no try.”



