Is this inhibitor the future of celiac disease treatment?

The efficacy of a novel therapeutic candidate for celiac disease has been validated at the molecular level, marking an initial step towards the first pharmaceutical intervention for the disease.
A European research collaboration led by Tampere University (Finland) has conducted a molecular analysis of the pathways involved in a therapeutic candidate for celiac disease. By validating the pathways involved and demonstrating the candidate’s efficacy at a molecular level, the team brings the field a step closer to the first pharmaceutical intervention for the disease.
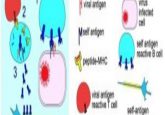
In 2% of the global population, cereals containing gluten can trigger an autoimmune response in the small intestine, a condition known as celiac disease. This results from a collection of molecular aberrations, such as the possession of one of two variants of the gene for human leukocyte antigen (HLA), HLA-DQ2 and HLA-DQ8, which are responsible for the presentation of antigens on the surface of mucosal antigen-presenting cells.
In people with celiac, gluten binds HLA-DQ2 or HLA-DQ8, causing an autoimmune response. One key pathway involved in this response results from autoantigen transglutaminase 2’s (TG2) deamidating specific glutamine residues on gluten, which increases recognition and presentation by HLA-DQ2 or HLA-DQ8. This presentation leads to the stimulation of T cells that attack essential structures of the intestine such as villi and crypt cells resulting in a host of symptoms from inflammation and diarrhea to anemia and type 1 diabetes.
There are currently no interventions for this disease beyond a gluten-free diet (GFD). What’s more, even in celiac patients who strictly avoid gluten, evidence has shown that markers of intestinal damage are still present and the expression of nutrient-absorbing proteins is reduced, suggesting that hidden gluten can still make its way into their diet.

Can AI predict the development of autoimmune diseases?
A new study details how an AI algorithm has been trained to identify genes associated with autoimmune diseases, hopefully allowing for earlier intervention.
To address this issue and provide a more comprehensive treatment strategy for celiac patients, the team set out to investigate a potential drug candidate. Previous studies have revealed that the TG2 inhibitor ZED1227 can prevent gluten-induced damage in people with celiac disease; however, its molecular impacts have not been revealed.
To elucidate this mechanism and the efficacy of the inhibitor at a molecular level, the team conducted transcriptional investigations of biopsies from a group of 58 celiac patients on a GFD before and after a 6-week challenge trial. The trial consisted of daily exposure to 3 g of gluten coupled with the prescription of 100 mg of ZED1227 or a placebo drug. RNA was extracted from PaxFPE-fixed biopsies using a Qiagen (Hilden, Germany) RNeasy kit and next-generation RNA sequencing was conducted using an Illumina (CA, USA) NextSeq sequencer to identify differentially expressed genes (DEGs) in the two cohorts.
Detailing the results of the study, senior author Keijo Viiri stated that by, “… measuring gene activity, we found that orally ingested ZED1227 effectively prevented gluten-induced intestinal mucosal damage and inflammation. In the drug group, the activity of the genes responsible for the absorption of nutrients and trace elements also returned to the pre-gluten exposure level.”
The team conducted further investigations of the DEGs identified in the placebo group after gluten challenge, using reactome and gene ontology enrichment analyses and comparing the DEGs identified to those induced when human intestinal organoids were exposed to interferon-γ. This revealed that almost half of the gene expression changes induced by gluten in the celiac patients were due to the intestinal epithelial response to the inflammatory cytokine interferon-γ, providing a deeper insight into the pathogenesis of celiac disease.
These results could hail the beginning of a new treatment paradigm for celiac disease, albeit one that is in its embryonic stages. However, despite the novelty of these discoveries, Viiri is excited by the potential of ZED1227, stating that, “it is a strong drug candidate that could potentially be used in conjunction with a gluten-free diet. If or when the ZED1227 medicine becomes available, it would also be useful to apply personalized medicine, especially to celiac patients with the high-risk HLA genotype.”