Toxin-inspired platform could improve the delivery of RNA therapeutics
Refer a colleague
The platform utilizes a modified diphtheria toxin and is capable of delivering RNA therapeutics for the modification of genes implicated in cancers and genetic diseases.
A team of researchers from the University of Toronto and SickKids Hospital (both Ontario, Canada) has developed a novel platform for the delivery of RNA therapeutics into cells to target aberrantly expressed genes.
RNA therapeutics work by controlling the expression of disease-implicated genes using either aptamers, silencing RNAs (siRNAs), microRNAs, synthetic mRNAs or antisense oligonucleotides. To modify these genes the molecules must interact with the cell’s DNA, however, there have been challenges with previous delivery methods becoming trapped in endosomes and being unable to fulfill this purpose.
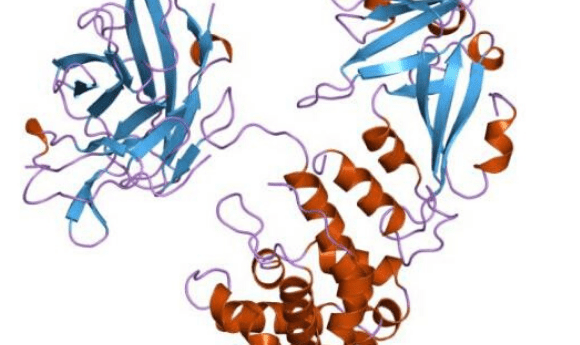
The bacterium Corynebacterium diptheriae produces a toxin that enters cells and triggers cell death, critically this toxin has the ability to escape endosomes. This characteristic inspired the repurposing of the toxin for the delivery of RNA therapeutics.
Study co-lead Roman Melnyk’s (SickKids Hospital) lab developed an attenuated diphtheria toxin, capable of escaping the endosome without infecting the cell.
The team then tested the concept by conjugating the attenuated toxin with siRNA targeted at two genes implicated in glioblastoma. Glioblastoma neural stem cells are thought to be resistant to traditional chemotherapeutics.
 Two minutes with: Joe Charest on organ-on-a-chip technology for drug development
Two minutes with: Joe Charest on organ-on-a-chip technology for drug development
We caught up with Joe Charest, Biomedical Solutions Program Manager at Draper (MA, USA), to discuss their work in ‘humanizing’ drug development with organ-on-a-chip technology.
The two genes selected were ITGB1 – which is associated with a highly invasive phenotype in many cancers – and EIF3B, which is known to play an essential role in cell survival.
“ITGB1 is involved in cancer cell migration, which contributes to glioblastoma’s invasion into healthy brain tissues,” explained study author Laura Smith (University of Toronto). “We used an innovative 3D culture system to significantly reduce cell invasion after treatment with our siRNA-attenuated diphtheria toxin system, which suggests that it may be effective in slowing disease progression.”
Significant downregulation of EIF3B was also observed, demonstrating the ability of this delivery platform to influence the survival pathway of cancer cells.
These findings have significant implications for the treatment of cancer and other genetic diseases. The team now hopes to test the platform in different disease types and with other RNA therapeutics.
Please enter your username and password below, if you are not yet a member of BioTechniques remember you can register for free.