Poised to attack

An epigenetic memory of a decade-old viral infection leaves human CD8 T cells ready to attack.

Human CD8 T cells defend against viral infection; their previous battles leave them epigenetically poised for attack.
Antibodies usually take center stage when discussing the body’s response to viral infection, but there is a second, perhaps more sinister, component necessary for establishing viral immunity: killer, effector CD8 T cells. Even when an antibody is effective, some virus still sneaks through to infect cells, and CD8 T effector cells target and kill the body’s own infected cells. Their response needs to be swift to prevent the spread of infection and the progression of critical symptoms.
A swift response to previously encountered pathogens depends on another type of CD8 T cell called memory CD8 T cells, which are poorly understood. Most studies on memory CD8 T cells are conducted in mice. In a recent study published in Nature, researchers investigated memory CD8 T cells in vivo in humans and found that their rapid response comes from a surprising epigenetic memory of previous infection (1).
“This is probably the first description at this level of detail and precision looking straight in humans,” said senior author Rafi Ahmed, director of the vaccine center at Emory University School of Medicine.
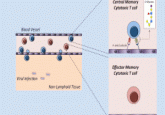
Ahmed’s team injected volunteers with a live yellow fever vaccine, a non-required vaccine that confers long-term immunity, and tracked CD8 T cells that proliferated in the blood after vaccination using deuterium labeling. Initially there was an increase in effector CD8 T cells in response to the infection, but this eventually decreased leaving just CD8 T memory cells. Previous studies showed the presence of memory CD8 T cells years after infection, but it was not known how often these cells divide, particularly in live humans. By quantifying deuterium kinetics, Ahmed’s team tracked cellular turnover rates and discovered that these cells are quiescent, dividing only once every year to year and a half, a surprisingly low division rate.
Ahmed team looked at the transcriptional and epigenetic profiles of memory CD8 T cells. Transcriptionally, the memory CD8 T cells were not much different from naïve CD8 T cells that had never encountered a virus. However, epigenetically, their profile resembled killer effector CD8 T cells. The chromatin structures of many of the genes that effector CD8 T cells express for controlling viral infections were open in memory CD8 T cells isolated a full decade after infection.
“There is an epigenetic memory of having seen an antigen ten years back. I think that was a very wonderful surprise, because it provided a mechanistic explanation for why memory cells respond faster,” said Ahmed. Even though the memory CD8 T cells don’t actively express the killer genes like effector CD8 T cells, they remained epigenetically poised to attack.
In the same issue of Nature, Ahmed’s team also published an accompanying article about work they performed in mice showing that the memory cells come from effector cells (2). Together, the two studies shed light on the often-debated question of whether or not memory cells actually express effector molecules.
Ahmed hopes to continue investigating the epigenetic profile of memory CD8 T cells. “We’re not really epigenetic aficionados like some other people are, but I think it’s a really important question for people to address how this epigenetic memory is maintained for so long. This is happening at very selective sites of the chromatin. To me that’s a very basic and fundamental mechanism question that needs to be addressed,” said Ahmed.
The team is also investigating if these findings are unique to yellow fever vaccination or if they are applicable to other infections and vaccines. Lastly, with future access to human autopsy material, Ahmed also hopes to expand the study from circulating CD8 T cells to resident tissue CD8 T cells.