Preparing the troops: cryo-EM image provides insight into T-cell activation
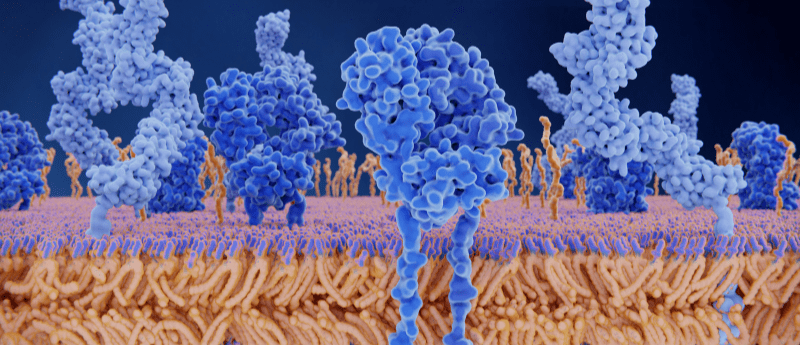
A recent study provides insight into how antigen binding primes T cells to be at the forefront of the immune response.
T cells are arguably one of the most crucial components of the immune system, fighting on the front line against foreign bodies including bacteria, pathogens and cancerous cells. Interactions between the highly specific T-cell receptor and antigens direct the full force of the immune system against invading pathogens only. Despite the dependence our immune systems have on T cells, it is relatively unknown as to how signaling events are activated following a T-cell receptor–antigen interaction. Researchers from the Institute of Biochemistry at Goethe University Frankfurt, University of Oxford and the Max Planck Institute of Biophysics were able to use cryo-EM to help answer this.
The research team hypothesized that binding of the antigen to the T-cell receptor would cause a conformational change, allowing it to initiate a downstream signaling cascade. By comparing an antigen-bound receptor image with that of an unbound receptor, the researchers could evaluate structural differences.
The team utilized a T-cell receptor commonly used in immunotherapy to treat melanoma, tweaked so that it could bind with its specific antigen as tightly as possible. Isolating the antigen-bound receptor prior to imaging proved difficult. Robert Tampé (Goethe University Frankfurt) commented on the novelty of the research area, reporting that “until recently, nobody believed that it would be possible at all to extract such a large membrane protein complex in a stable form from the membrane.”
Subsequent cryo-EM imaging of the complexes that had survived the preparation process revealed insights into the T-cell receptor activation mechanism. Tampé stated: “On the basis of our structural analysis, we were able to show how the T-cell receptor assembles and recognizes antigens and hypothesize how signal transduction is triggered after antigen binding.”
 Sound the alarm: CD8+ regulatory-like T cells are first on the scene of a stroke
Sound the alarm: CD8+ regulatory-like T cells are first on the scene of a stroke
A subtype of lymphocyte responds rapidly to stroke onset in mice, providing neuroprotection to the brain.
Their findings, published in Cell, indicate that there is no significant conformational change in the receptor structure following antigen binding. This poses a follow up question. If antigen binding does not impact T-cell receptor structure, how does it trigger T-cell activation? The research team suggest that the CD8 receptor may also play a crucial role, by phosphorylating the intracellular portion of the T-cell receptor. In the absence of phosphatases, the phosphate groups are therefore able to direct the next stage in the cell signaling cascade.
“Our structure is a blueprint for future studies on T-cell activation. In addition, it’s an important stimulus for employing the T-cell receptor in a therapeutic context for treating infections, cancer, and autoimmune diseases.,” concluded Tampé.