microRNAs as a diagnostic and prognostic biomarker for psychiatric disorders
 Cecilia Flores (left) is a Professor in the Department of Psychiatry at McGill University (Montreal, Canada), where she runs a lab focused on the adolescent brain and the mechanisms underlying its plasticity. Through her study of this plasticity, she hopes to improve our understanding of adolescents’ increased vulnerability to psychiatric disorders, such as depression.
Cecilia Flores (left) is a Professor in the Department of Psychiatry at McGill University (Montreal, Canada), where she runs a lab focused on the adolescent brain and the mechanisms underlying its plasticity. Through her study of this plasticity, she hopes to improve our understanding of adolescents’ increased vulnerability to psychiatric disorders, such as depression.
 In this interview, Cecilia is joined by her PhD student, Alice Morgunova (right), whose thesis explores the biological markers that could be used to assess the risk of an individual developing psychiatric disorders. In conversation with Digital Editor Annie Coulson at this year’s meeting of the Society for Neuroscience (SfN), Neuroscience 2023 (11–15 November; Washington DC, USA), Cecilia and Alice discussed their work on the molecular and cellular pathways that underlie depression in adolescence, and explained the techniques utilized to accurately profile biomarkers and determine the differences between healthy control individuals and adolescents diagnosed with depression.
In this interview, Cecilia is joined by her PhD student, Alice Morgunova (right), whose thesis explores the biological markers that could be used to assess the risk of an individual developing psychiatric disorders. In conversation with Digital Editor Annie Coulson at this year’s meeting of the Society for Neuroscience (SfN), Neuroscience 2023 (11–15 November; Washington DC, USA), Cecilia and Alice discussed their work on the molecular and cellular pathways that underlie depression in adolescence, and explained the techniques utilized to accurately profile biomarkers and determine the differences between healthy control individuals and adolescents diagnosed with depression.
What are you presenting at SfN 2023?
Alice: The project that I’m presenting at SfN focuses on depression in adolescence. We non-invasively profiled peripheral markers in blood, focusing on microRNAs, while using advanced techniques to accurately create and analyze the microRNA profiles for individuals with depression and healthy control groups. By assessing the differences between the control and study groups, we have been able to identify some biomarkers that can potentially be used as diagnostic or prognostic markers for depression.
Cecilia: We are particularly interested in microRNAs due to their ability to target several genes at a time and to inhibit or repress gene activity, making them really powerful. By establishing profiles of these microRNAs and conducting bioinformatics investigations on them, in conjunction with the work that we have done in rodents, we’re learning about the function that these microRNAs might have in the brain. Not only are we able to detect who is diagnosed with depression and who is not, but we can also learn a little bit about the possible mechanisms that are involved in this disorder.
What techniques did you use in this project?
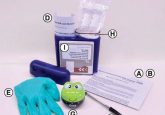
Cecilia: This project utilized longitudinal cohorts of adolescents maintained by our collaborators across the University of California, Los Angeles, Stanford (both CA, USA) and McGill. They obtain dry blood spot samples from these cohorts, which involves collecting a droplet of blood on a sheet of paper. This technique is fantastic as it’s noninvasive, inexpensive and requires very little storage space. We took a little bit of the paper card with the preserved blood and performed sequencing for microRNA to create a profile for each subject’s microRNA expression.
Alice: The dried blood spot sampling method is decades old and is commonly used in newborns due to its minimal invasiveness and for routine infectious disease or metabolic testing in adults. In the past, it would have been challenging to leverage these samples for microRNA profiling, due to the small sample input volume; however, by collaborating with labs that use a sequencing technique with very low input requirements, we are able to create these microRNA profiles.
The beautiful thing about microRNAs is that they are small, only a few nucleotides long, and remarkably stable in those samples, which allows us to capture these profiles while making it as minimally invasive for the participants as possible.
What challenges did you face on this project?
Alice: In our pilot, everything went seamlessly and we were very excited. But when we were processing the whole cohort, over 60 individuals’ samples, the company we were using to standardize the processing pipeline said the samples’ quality was inadequate to the point of being unusable and we would have to find other samples; this wasn’t really an option. However, we were determined to make this work.
So, together we decided, “you know what? We’re going to take these samples and figure out the processing pipeline ourselves.” We were creative and used some other techniques. For example, to quantify RNA in order to ensure equal input of the samples for each sequencing run, you typically use the total RNA. But RNA is usually present in large chunks, such as the ribosomal or messenger RNA, which do not survive well in these dried blood spots, so you can’t equalize the samples with the typical nanodrop-based metric. Instead, we used a modification of a technique involving fluorescent dyes, called Quant-iT RiboGreen RNA quantification, to accurately portray the small quantity of the RNA that is in those samples. Once we saw that we still had our RNA, we did an initial pilot sequencing and it produced robust data. We saw that, in fact, the largest proportion of RNAs present were microRNAs rather than other small RNA types, which was ideal for our purpose.
Cecilia: In summary, we were dealing with very small amounts of RNA, which the company that typically does the sequencing for our samples couldn’t deal with. So, we managed to work with very small inputs by ourselves, borrowing methods that are typically used to sequence microRNAs that are inside vesicles.
Alice: Another key challenge was with the computational processing. When we were doing computational analysis, prior to aligning the sequences to the human genome, we had to do a few filtering steps. The library preparation that we use employs adapter sequences with degenerate sequences, which are three random nucleotides at the end of the adaptors that enable them to bind to microRNA sequences without any bias. While this eliminates the bias from your screen, it also essentially embeds the adapter sequence and three random nucleotides prior to the sequence of interest, making it a little confusing to identify what is part of the sequence of interest and what’s the adapter sequence. To overcome this, it is important to modify some of the processing pipelines during the computational analysis to specify exactly which sequences are the adapters.
What’s next for this project?
Cecilia: We started the study with this adolescent cohort of subjects who have been diagnosed with depression versus healthy controls. The most important next step is to replicate our findings in an independent cohort. We already have preliminary microRNA sequencing data from a community cohort of children in Singapore. These data were extracted from blood plasma samples as opposed to dried blood spots and the results are already very interesting; we think we have identified a common microRNA that is dysregulated in both samples. Essentially, our next step is to replicate and then use these data to establish microRNA profiles of adolescent development in subjects without a psychiatric disorder.
Alice: Within the same subjects, we’re also interested in looking closer at their genotype, which will allow us to assess genetic variations at the nucleotide level within these individuals and create polygenic risk scores based on those single variants. This should allow us to select genes to explore further, developing an understanding of their function and their regulation by microRNA, which I find incredibly exciting. In summary, we want to be able to draw information from microRNA on multiple levels, all the way from peripheral microRNA, to predicting which genes may be active or modulated by these microRNAs in the brain.
Is there any therapeutic potential for the microRNAs you identified?
Cecilia: I definitely think so but it would be too early to switch to investigating therapeutic avenues. However, we are always moving from identifying microRNA biomarkers in humans to investigating their function in rodents. We have manipulated microRNAs directly in the brain of mice and have found corresponding changes in blood and in their behavior, for example, reducing the risk of stress-induced behavioral disorders in these mice or, in other cases, increasing this vulnerability.
Based on these observations, we’re trying now to develop molecules that would regulate these microRNAs, delivering them by intranasal administration. We have a poster discussing this work at SfN as well, alongside another discussing the direct delivery of microRNAs in vesicles to the brain of mice in order to induce behavioral changes. So, we are not there yet, but there are projects in progress to explore this capability.
What impact do you think these biomarkers could have in the management of mental health disorders?
Cecilia: Everything started with our work in rodents, measuring microRNA in blood in adolescent mice and using it to predict the severity of their response to stress later on. I think the noninvasive method we used for this could prove useful in both diagnosing depression in adolescents and predicting future symptoms or determining risk. This could be hugely influential in informing prevention and early intervention strategies for adolescents.
Alice: That’s exactly it for me as well. My project is very much looking at this global microRNA profile during the developmental period of life and how all these biomarkers may be implicated in disease. Based on these identified candidates, the continuation of this work in Cecilia’s laboratory will use rodent models to directly measure and manipulate these markers in the brain and study the pathways implicated by these biomarkers in psychiatric disorders.
If you would like to find out more about the methods discussed in this interview, you can view Cecilia and Alice’s article, “Preparation and processing of dried blood spots for microRNA sequencing” here.