Auto-secretion of heterologous proteins from E. coli

Researchers have discovered that a green fluorescent protein variant can be used as a tag to enable the auto-secretion of heterologous proteins expressed in Escherichia coli.
Large-scale production of recombinant proteins in Escherichia coli occurs through either intracellular or secretory expression. The latter route is advantageous since the former requires a cell lysis step that increases the complexity and cost of the protein isolation process.
Protein secretion from E. coli typically proceeds through several different signal peptide-mediated secretion systems, but there are unconventional secretion pathways that do not involve a signal peptide. Now, researchers led by Lixin Ma at Hubei University describe their discovery of such a pathway for super-folder green fluorescent protein (sfGFP) overexpressed in E. coli and how they exploited this pathway to enable the auto-secretion of heterologous proteins from E. coli.
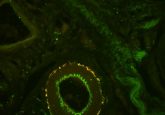
sfGFP (~26 kDa) is a mutant of GFP engineered to have brighter fluorescence, better solubility, faster folding, and greater resistance to denaturation. Ma’s group accidentally discovered that sfGFP overexpressed in E. coli was auto-secreted into the culture medium. Microscopy revealed green fluorescence in the cytoplasm, periplasmic space, and in the outer membrane, indicating that sfGFP was properly folded throughout this extracellular secretion pathway.
Sequence analysis of sfGFP revealed no potential signal peptides and the N-terminus of the secreted sfGFP was intact, suggesting that its secretion does not depend on a signal sequence. Deletions of the N-terminal portion of sfGFP, which is important for its β-barrel structure, reduced the secretion of sfGFP, suggesting that the β-barrel plays a major role in the process. The overall negative charge of sfGFP is also critical; changing the net charge of sfGFP to very positive greatly reduced secretion.
Based on the auto-secretion of sfGFP, the team developed a method to enable the secretion of heterologous proteins expressed in E. coli by fusing them to the C-terminus of sfGFP. They tested this new method, named E. coli sfGFP mediated protein secretion system (EGPSS), with proteins ranging in size from polypeptides to multi-protein complexes.
A monomeric 29 kDa enzyme (endo-β-N-acetylglucosaminidase H) was successfully secreted into the culture medium with its enzymatic activity intact. For human arginase-1 (ARG-1; 36 kDa), a larger and more complex enzyme that only has enzymatic activity as a homotrimer and typically forms inclusion bodies in E. coli, EGPSS enabled 10% of the total expressed sfGFP-ARG-1 to be secreted in a soluble form while maintaining enzymatic activity. The researchers observed expression of sfGFP-ARG-1 in the outer membrane, and interestingly, the whole cells expressing sfGFP-ARG-1 demonstrated arginase activity, suggesting that they could be used as bioreactors. Finally, they used EGPSS successfully for the secretion expression of enzymatically active glutamate decarboxylase (GAD; 51 kDa), which forms a homohexamer in its active state.
EGPSS allowed easy monitoring of the expression level of the secreted fusion protein by quantifying the fluorescence of the culture medium, and the team demonstrated that it is well-suited for industrial scale production of heterologous proteins from E. coli in fed-batch cultures.