Bridging the divide
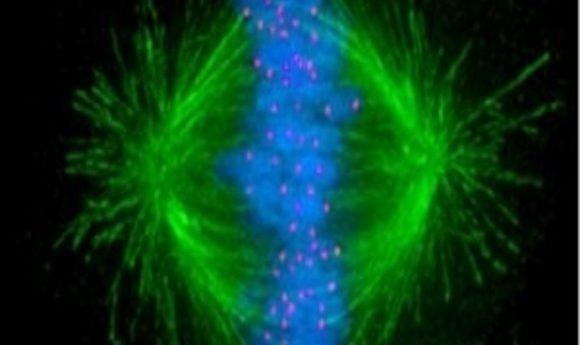
New microscopy techniques offer an unprecedented view of chromosome separation during cell division, revealing new details of activity at the kinetochore.
If chromosomes aren’t properly distributed during cell division, a host of potential issues—ranging from cancer to infertility to developmental defects—may result. Researchers have a very clear overview of this process, yet some of the intricate details of how chromosomes pull apart during division remain elusive.
In 2013, Viji Draviam and her colleagues at Queen Mary University of London (QMUL) visualized the multi-step process involved in capturing chromosomes at the kinetochore in human cells for the first time. But despite this advance, they weren’t able to determine the master regulators governing the process.
“Historically, kinetochore microtubule attachments were studied in detail by electron microscopy. The kinetochore is a submicron structure, and the microtubules are only 23 nanometers in width, so it was the only tool we could really trust,” she explained. “But it’s a fixed-cell technique that only gives you a snapshot of what’s happening; it doesn’t allow you to look at the dynamic processes. The microtubule grows and shrinks quite dramatically during cell division, which is what moves the chromosomes. The question we wanted to ask was whether cells were moving normally or not, which was hard to determine with a fixed technique.”
Recent advances in microscopy now allow the high-resolution, live-cell imaging at very high speeds needed for this purpose. Draviam and her colleagues turned to super-resolution microscopy and high-speed, time-locked microscopy techniques to look at the movement of the microtubules during human cell division. In doing so, they identified two key proteins, Aurora-B kinase and BubR1-bound PP2A phosphatase, that work in opposition to add or subtract phosphate groups from the microtubules. In doing so, they regulate chromosomal attachment during this vital process.
“This is a further step in understanding these very intricate mechanisms that human cells use to protect their chromosome numbers. When we can understand the normal, correct mechanisms, we can start to better understand what’s going wrong in pathologies,” said Draviam.
With the regulators in hand, Draviam’s team is interested in teasing out the rest of the players involved and the roles they play in healthy cell division. “There’s still quite a bit to understand. Our next step is to figure out the downstream components of this pathway,” she said. “By visualizing the molecular changes in actual cells, we can move closer to being clinically useful, understanding how chromosomes are captured, and perhaps, one day, being able to go directly to clinical tissues to understand if those changes were made at the correct time in different patient groups.”





