Picture perfect: imaging the full 3D orientation and position of molecules in cells

This novel hybrid microscope allows for the simultaneous imaging of the full 3D orientation and position of molecules within cells.
Researchers from the Marine Biological Laboratory (MA, USA) have developed a way to simultaneously image the 3D orientation and position of molecules in a cell, which can provide insight into protein interactions and cell division. They did this by combining existing imaging technologies, offering greater insights than either technology individually.
Cellular molecules regularly rearrange themselves to create dynamic 3D structures that inform function. Previous microscopy techniques are limited; they are only able to measure a subset of orientation parameters. This inspired the current team to develop a microscopy technique that can assess the complete 3D orientation and position distribution of multiple fluorescent molecules in a cell.
Using the molecules in the spindle of a dividing cell as an example, co-author Rudolf Oldenbourg explained: “With traditional microscopy, including polarized light, you can study the spindle quite nicely if it’s in the plane perpendicular to the viewing direction. As soon as the plane is tilted, the readout becomes ambiguous.” The novel microscopy technique can correct for tilt while capturing the 3D orientation and position of the microtubules.

In conversation: Michelle Itano on ASCB 2024 and the latest developments in immersion microscopy
In this interview feature, we catch up with our Editor-in-Chief, Michelle Itano to discuss her recent experiences at the annual meeting of the American Society for Cell Biology.
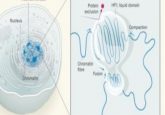
The hybrid microscope combines polarized fluorescence technology, a tool that can measure molecular orientation, and dual-view light sheet microscopy (diSPIM), which is specialized to image along the depth axis of a sample.
In more detail, the diSPIM microscope component has two imaging paths that meet at a right angle in the sample, providing two different views of the sample that can compensate for poor depth resolution. The researchers realized that the dual-view microscope could also address a limitation of polarized light microscopy, which is that it’s difficult to efficiently illuminate the sample with polarized light along the direction of light propagation. The team then modified the diSPIM with liquid crystals, allowing them to change the direction of input polarization.
In the future, the team wants to make their microscopy technique faster so they can observe how molecules’ position and orientation change over time in live samples. They are also hopeful that the range of fluorescent probes will be expanded, facilitating the imaging of a greater variety of biological structures.