Seeing DNA clearly now
A gentle new technique allows scientists to see the fine structure and 3-D organization of individual strands of chromatin in living cells. It turns out that the textbooks are wrong.
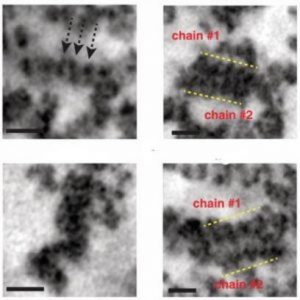
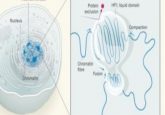
Images of different chromatin structures (1).
A while back, when Clodagh O’Shea was working on a viral protein that specifies heterochromatin silencing of p53 and other genes, she hit a snag. Histone marks associated with this silencing, but they did not explain why the genes were dominantly repressed and inaccessible to p53 protein. Since structure determines function, O’Shea realized she needed to better understand the structure of normal chromatin before she could understand silenced chromatin, so she turned to textbooks. The diagrams she found of chromatin folded in a regular pattern fit neither her observations nor the wide range of gene expression and epigenetic inheritance profiles that exist in cells.
“It didn’t make sense that there would be only a limited set of discrete hierarchical folded structures because that would suggest that there would be only a corresponding number of discrete gene activity outputs and states,” said O’Shea, now at the Salk Institute. So, she and her group decided to come up with a new method to visualize chromatin structure, which they recently published in Science.
Each tiny cell nucleus contains 2 meters of DNA. The standard theory dictates that to pack that all into the nucleus, 147-bp sections of double helix DNA winds around octamers of the histone proteins H2A, H2B, H3, or H4 to make 11-nm nucleosomes, which then fold around histone H1 to make a 30 -nm-wide DNA “wire,” which is then wound upon itself to make a 120–300-nm-wide uniform fiber.
But, “the hierarchical folding model is a cartoon that is largely based on the structures of chromatin either formed in vitro by dilute, highly purified, and uniform populations of DNA and nucleosomes, or in nuclei where other components have been extracted,” said O’Shea. “And so, quite frankly, we thought that was a bit of a problem. It’s really quite important to understand what the structure of chromatin is in the context of intact cells.”
No method had been able to unambiguously visualize how DNA is actually packaged in a whole, living cell. Heavy metals stain parts of cells to provide contrast for EM, but these stains bind lipids, proteins, and RNA more strongly than DNA. Other dyes require harsh acid treatment of the cells, which destroys chromatin structure. So, Horng Ou in O’Shea’s lab used a cell-based assay to screen fluorescent dyes for their ability to photooxidize diaminobenzidine (DAB).
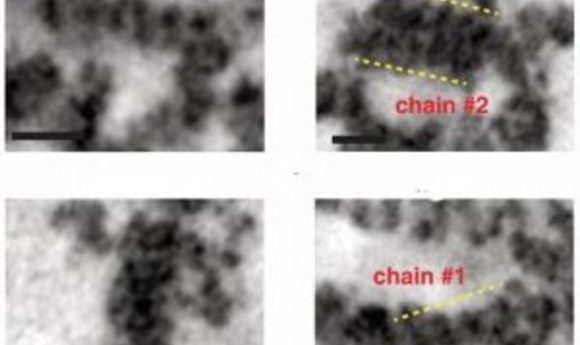
They found one dye called DRAQ5(TM; BioStatus Limited) that bound to DNA and whose excitation photooxidized DAB, catalyzing the deposition of DAB polymers on the surface of the DNA. Osmium tetroxide (OsO4) which can be visualized by EM, binds to these DAB particles, highlighting the chromatin. Sebastian Phan from the University of California, San Diego then used advances he had made in multi-tilt EM tomography to visualize and reconstruct megabases of individual chromatin chains, heterochromatin, and euchromatin as a continuum in the nucleus. The team call their new technique ChromEMT.
When they applied ChromEMT, the researchers found that chromatin looked a lot different from textbook models: it was a granular chain that looped and folded into a variety of different structures, such as helices, stacks, and loops, in different densities and with a range of fluctuating diameters between 5 and 24 nm. Chromatin is a disordered and flexible chain, and its primary polymer structure doesn’t change during mitosis, which helps explain how epigenetic structures and interactions could be passed on to the next generation. Disordered chromatin is more flexible and can bend to be more or less dense, which can encourage or inhibit gene transcription.
“I had a sense of awe when I saw it for the first time,” said O’Shea. “I thought, wow! This is the first time anyone has seen the chromatin and 3-D structure of the human genome.”