Bacteria’s unique mechanism for antiviral immunity
A mechanism of antiviral immunity in bacteria has been characterized.
A research team from Umeå University (Sweden) has uncovered a mechanism for how bacteria build up antiviral immunity against bacteriophages, separate from established anti-phage defense systems. This discovery could open new avenues for combating antibiotic resistance.
Using viruses to target bacteria has been suggested as a key approach for combating antibiotic resistance; however, before this approach can be utilized, the mechanisms underlying bacteria’s defense against viruses must first be understood.
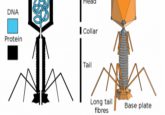
Bacteria, which are susceptible to infection by bacteriophages, often carry multiple genes encoding anti-phage defense systems, providing them with antiviral immunity. These defense systems are ubiquitous among bacteria and are clustered in defense islands and phage parasites.
The prototypical family of phage parasites from Staphylococcus aureus, known as Staphylococcus aureus Pathogenicity Islands (SaPIs), parasitize phage by enlisting a transcriptional repressor, Stl. Stl not only suppresses SaPI genes but also senses a phage infection by binding to the phage-encoded anti-repressor protein – which is SaPI-specific.
 Coming of phage: treating antibiotic-resistant infections with bacteriophages
Coming of phage: treating antibiotic-resistant infections with bacteriophages
In this feature: phages’ diverse structure and function, the roles that both natural and engineered phages could play in overcoming the growing issue of antibiotic-resistant bacterial infections and what happens if bacteria become phage-resistant.
However, this feature does not fully explain the ubiquitous nature of phage parasites, leading researchers to believe that bacteria have further antiviral mechanisms. Furthermore, there is a growing body of evidence to suggest that bacteria have antiviral systems that target phage-encoded homologous recombinases (HRs) – proteins essential to phage replication.
To investigate this, the team studied the S. aureus bacterium, a subgroup of which has become multi-resistant to antibiotic treatment. Through comprehensive analysis of cryo-electron microscopy structures, the researchers were able to show a mechanism for antiviral immunity in bacteria that targeted these HRs.
While SaPIs may possess anti-phage defense systems, they can also provide strong immunity through their conserved transcriptional repressor, Stl. The team demonstrated how Stl2, the SaPI2-encoded Stl, targets and inhibits phage-encoded HRs by forming divergent ring- or filament-like structures that exhibit inhibitory activity. Consequently, the phage’s ability to replicate DNA is blocked, preventing it from infecting more bacteria.
The researchers also noted that this work supports the idea that phage parasites were naturally selected as bacterial defense mechanisms due to the conservation of Stls in these parasites, helping to explain why they are highly ubiquitous across microbiomes.