Critical role of nSMase2 in HIV replication reveals new antiretroviral target
A new antiretroviral target has been identified that suppresses HIV-1 replication and selectively kills HIV-1-infected cells.
A collaboration of researchers led by Johns Hopkins University and the US NIH (both MD, USA) has published two studies reporting the critical role of an enzyme, neutral sphingomyelinase-2 (nSMase2), in the late stages of HIV-1 replication and maturation. They developed a new class of antiretroviral compounds that blocks the enzyme and reported it both suppresses HIV-1 replication and kills infected cells in human cell lines and mouse model studies.
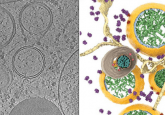
HIV-1 is the most common type of HIV. When HIV-1 leaves infected cells, particles bud off from the surface of the infected cell and a protective viral envelope is produced from the plasma membrane. “The HIV genome is very small, so to replicate itself, it hijacks all sorts of cellular machinery. nSMase2 is one piece of this hijacked machinery that we found is absolutely essential for HIV to properly replicate itself,” commented Norman Haughey (Johns Hopkins Medicine), a co-corresponding author of both studies. Both studies focus on the creation of the viral envelope to gain further understanding of how HIV-1 replicates, in the hopes of identifying a new therapeutic approach for treating HIV-1 infection.
 Structure of enzyme involved in pre-tRNA splicing revealed
Structure of enzyme involved in pre-tRNA splicing revealed
Researchers at the Goethe University use cryo-electron microscopy to study a key enzyme involved in protein production.
The first study builds on previous observations that nSMase2 breaks down complex fats in cell membranes into a simpler form called ceramides. They found that ceramide is essential in the final stages of HIV-1 reproduction, providing a protective coating for new virus particles, demonstrating that nSMase2 is a necessary cellular cofactor in the late stages of HIV-1 replication. [1]
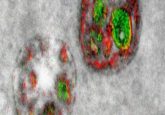
The second study investigated the inhibition of nSMase2 in HIV-1-infected mice with humanized immune systems using an experimental nSMase2 selective inhibitor called PDDC. Electron microscopy showed that blocking or deleting nSMase2 resulted in misshaped virions, which did not mature and were not infectious and eventually resulted in the death of infected cells.
In mice, PDDC reduced HIV-1 levels in blood to undetectable amounts, achieving comparable levels to treatments currently used to manage HIV. Unlike current antiretrovirals, when treatment with PDDC was stopped, 80% of the mice did not experience a rebound in HIV. Results from in vivo and tissue culture experiments showed that PDDC selectively kills cells with actively replicating HIV-1. [2]
The papers show that targeting nSMase2 in a cell that is making HIV-1 changes the lipid composition of the virus particle, thereby preventing virus maturation and virus infectivity. nSMase2 is the first antiretroviral target that has been identified as capable of killing infected cells and has the potential for a new therapeutic approach to treating HIV-1 infection.
“We still have a long way to go, this is just a piece of the puzzle,” concluded Eric Freed (National Cancer Institute, MD, USA), co-corresponding author of both studies. “This enzyme is one foot in the door to better understanding the viral lipid composition in the late stages of the replication cycle.”