First US clinical trial for intravenous phage therapy gets FDA approval
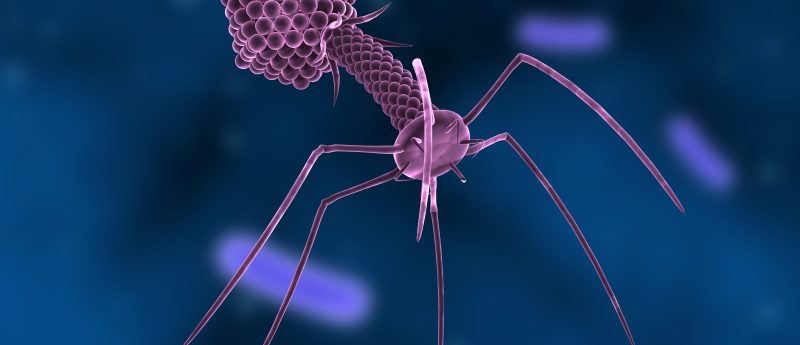
A team from the University of California San Diego School of Medicine (CA, USA), in collaboration with biotech company, AmpliPhi Biosciences Corporation (CA, USA), have been given the go ahead for a clinical trial using bacteriophage-based therapy, delivered intravenously, from the US FDA. The clinical trial will evaluate an experimental bacteriophage, AB-SA01, for treatment of approximately 10 participants, all suffering with resistant Staphylococcus aureus infections in ventricular assist devices (VADs). VADs, electromechanical implants that assist with cardiac pumping for functional circulation, are usually given to patients with heart weakness or failure in need of a transplant. The phage will be...
To view this content, please register now for access
Join our member community for FREE to access a collection of journal and online-only features, including:
- Exclusive access to educational videos, eBooks and insights into top BioTechniques journal articles
- The latest news and journal updates delivered straight to your inbox when you want it
- Personalized recommendations for the latest member-exclusive podcasts, interviews and expert opinions
- Priority registration to webinars, panel discussions and events
- Access to competitions and journal publication discounts, including 10% off open access fees when you sign up today!