Glowing squid, sticky bacteria, and the evolution of symbiosis
A laboratory evolution experiment involving squids and bioluminescent bacteria sheds light on the origins of bacterial symbiosis.

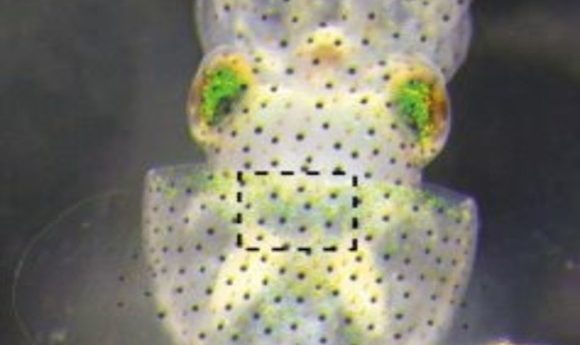
Hawaiian bobtail squid and its light organ (1).
Bacteria can be our best friends or our worst enemies. As mutualistic symbionts, they help us survive, and as opportunistic pathogens, they make us suffer. In a world where the friend so closely resembles the enemy, how can a host ensure that its immune defenses against pathogens do not keep out friendly bacteria? A new study from Cheryl Whistler and colleagues at the University of New Hampshire, Durham provides answers to this question, which the team discovered by allowing bacteria to evolve under natural conditions in a very unique model system.
The Hawaiian bobtail squid inhabits shallow oceans in the central Pacific. In order to escape predators at night, it employs a unique camouflage system. Symbiotic bacteria of the species Vibrio fischeri inhabit specialized light organs in the squid’s mantle where the bacteria emit light in the form of bioluminescence that appears like moonlight when viewed from below, hiding the squid’s silhouette. Both bacteria and squid benefit from this association; the light-based camouflage lets the squid avoid predators, and the bacteria get plentiful food to grow and reproduce.
However, not all strains of Vibrio fischeri share this capacity for friendly coexistence with their squid hosts. Some strains are free-living, while others find themselves better suited to other host species, such as fish. The squid themselves undergo daily cycles of purging and repopulating bacteria from their light organs, letting only bacteria that are highly skilled at colonization build up over time.
“The squid are very specific as to who they will let in their light organ, and we don’t fully understand how they can tell the difference between a symbiont and a non-symbiont,” said Whistler. “So it serves as a model for understanding how a host might produce the right barriers that the good bacteria can get past, but the pathogenic bacteria get blocked.”
Whistler and colleagues allowed five different strains of Vibrio fischeri isolated from wildly different environments to grow within squid light organs in the laboratory for several generations to see if the bacteria would eventually evolve to become better symbionts. And this indeed appeared to be the case. While colonizing several squids in succession over a few days, the populations of bacteria in the squid light organ began to evolve in such a way that the frequencies of certain mutations gradually increased in the population, which in some cases gave them a major competitive advantage for colonization when compared to the parental strains.
Surprisingly, over the course of the experiment, the researchers found that several successful bacterial colonists accumulated point mutations in the gene encoding a protein known as BinK. BinK is a global regulator of several cellular processes, and this result pointed at a fundamental mechanism via which hosts could exert selective pressures on potential symbionts.
The mutated binK allelle helped the bacteria become better colonists by improving their aggregation, helping them evade immune cells, and affecting quorum sensing (how they communicate with each other to adjust bioluminescence levels). The mutations accomplished most of this by promoting production of bacterial polysaccharides that create a sticky “sugar coating” that hides the bacteria from the squid’s immune system.
“It really has helped us understand more about the traits that allow bacteria to basically communicate to their hosts that they are the right symbiont,” said Whistler. “This gives us a lot of insight into how bacteria are so incredibly adaptable.”
“I think the approach they took is very elegant,” said Mark Mandel, an evolutionary biologist from the Feinberg School of Medicine, Northwestern University, who was not associated with the study. “I think by doing this in-squid evolution approach, they are able to learn a lot about the colonization process. I thought that’s a really nice combination of taking natural strains and letting the evolution tell the investigator what is important.”