No food for 2 days keeps the doctor away: the impact of fasting on infection

Fasting in mice impacts the strength of a gastrointestinal infection and the subsequent damage caused, reveals a new study highlighting how the microbiome links fasting and infection.
A recent mouse model study from the University of British Columbia (Canada), led by Bruce Vallance, has revealed that fasting impacts the virulence of gastrointestinal infection with a common cause of food poisoning. The work answers long-held questions about the impact of illness-induced fasting and provides an insight into the potential of therapeutic fasting.
It is a well observed phenomenon that when people are ill, particularly with gastrointestinal issues, they lose their appetite. This behavior is so inherent that many a casual observer would not think to question its impact or reason. However, in disease research, the debate rages as to whether fasting increases susceptibility to infection or protects against it. This debate hinges on whether fasting restricts the energy required by an active immune system, making it less efficient, or prevents the pathogen from accessing the nutrients needed to proliferate.
To investigate this, the team fasted a cohort of mice for 48hrs before oral infection with the bacterium Salmonella enterica serovar Typhimurium. Fasting was continued throughout the course of infection.
The fasted mice experienced significantly reduced proliferation of Salmonella in their intestines and the bacteria were not able to penetrate the intestinal wall, leading to decreased inflammation and tissue damage. On refeeding the infected fasted mice, proliferation of the Salmonella increased alongside invasion of the intestinal wall.
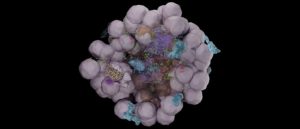
Electron microscopy reconstruction of the various bacterial niches. CREDIT: Sharma et al. © 2021 EPFL
Investigating how UTIs recur with organoid and organ-on-a-chip technology
Two new tissue models have been developed to help researchers investigate UTIs in the bladder. The models provide a new insight into the behaviors of the causative bacteria, Escherichia coli (E. coli), that help infer them with antibiotic resistance, leading to UTI recurrence.
To assess the impact of the gut microbiome in this observation, the team repeated the experiment in gnotobiotic mice – mice bred to contain no microbiome – and found that the fasting did not prevent infection to the same extent, although inflammation was still reduced. Further experiments with a different bacteria (Campylobacter jejuni) repeated the results, indicating that the impact of fasting was not specific to Salmonella.
Commenting on the results, the team explained that “Our research highlights the important role that food plays in regulating interactions between the host, enteric pathogens and the gut microbiome. When food is limited, the microbiome appears to sequester the nutrients that remain, preventing pathogens from acquiring the energy they need to infect the host. While more research is needed, fasting or otherwise adjusting food intake could be exploited therapeutically to modulate infectious diseases in the future.”
While these results need to be repeated and validated in humans, this study clearly highlights the potential of therapeutic fasting and the importance of the gut microbiome in protecting against bacterial infections, revealing new avenues for research into infectious diseases.