Parasites of the deep: bacterial invasion of mussels’ nuclei
A bacterial parasite can infiltrate the nuclei of deep-sea mussels, take control of the cell and reproduce to over 80,000 cells, all while the host is alive. Now, we know how.
Researchers at the Max Planck Institute for Marine Microbiology (Bremen, Germany) have discovered how a bacterial parasite colonizes the nuclei of deep-sea mussels. This study sheds light on the previously unknown cellular and molecular processes that these intranuclear parasites utilize to thrive off their animal hosts, expanding our understanding of host–microbe interactions.
Very few bacteria can inhabit cell organelles, but the bacterial parasite Candidatus Endonucleobacter has figured out how to do just that. Infecting the nuclei of mussels from cold seeps or hydrothermal vents around the world; a singular bacterial cell penetrates the nucleus and reproduces until at least 80,000 bacterial cells are swelling the nucleus to 50 times its original size. A common cellular response to this sort of parasitic invasion is apoptosis, which signals host cell death.
The mechanisms that enable this staggering phenomenon are yet to be understood, presenting an interesting conundrum for the microbiology community. In order to close this gap in our understanding, the researchers set out to discover how the microbes fuel themselves for such a reproductive feat while keeping the host cell from inducing apoptosis.
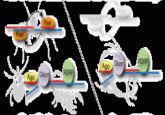
 Malaria parasite uses decoy proteins to distract the immune system
Malaria parasite uses decoy proteins to distract the immune system
The first high-resolution map of proteins in the malaria parasite Plasmodium falciparum suggests that the parasite uses decoy proteins to limit our long-term immune response.
To investigate this question, the team used an array of molecular and imaging techniques. They isolated infected nuclei using laser-capture microdissection and conducted dual RNA sequencing, which revealed that Candidatus Endonucleobacter doesn’t use nuclear DNA or RNA as its main fuel source. Instead, it upregulates genes that import and digest sugars, amino acids, and lipids from its host.
They also found that it is most likely able to prevent host-cell apoptosis by upregulating several inhibitors of apoptosis (IAPs), proteins that have not been observed in bacteria. A race for cellular control ensues; as the bacteria produce more IAPs, the host cell kicks its production of apoptosis-inducing proteins into high gear. Once the parasite has reproduced enough, the host cell ruptures, and the parasites are free to infect new nuclei. Comparative phylogenetic analyses revealed that this intranuclear parasite likely acquired IAPs from its hosts via horizontal gene transfer, which was assumed to be a rare phenomenon between eukaryotes and bacteria but may be more common than previously thought.
This study contributes to our understanding of the host–microbe relationship, potentially having broader implications for studying parasitic infections and the strategies they have evolved to thrive in host cells. “Our research sheds light on an overlooked mechanism of genetic exchange — HGT from eukaryotes to bacteria — potentially influencing how we understand microbial evolution and pathogenesis. Moreover, our study offers insights into apoptosis regulation, which is relevant to cancer research and cell biology,” concluded Nikolaus Leisch, co-senior author of the paper.